|
Uncaria tomentosa (Willd) DC. / Uncaria guianensis (Aubl.) J.F. Gmel.
INTRODUCTION Uncaria tomentosa (Willd.) DC. (1830) and Uncaria guianensis (Aubl.) J. F. Gmel. (1796), Class Dicot, Order Gentianales Family Rubiaceae. It is important to clarify that there are no ethnomedicalstatistical studies about the differentiated use of both species in traditional medicine for the treatment of certain diseases; thus, information should be observed under this criterion. |
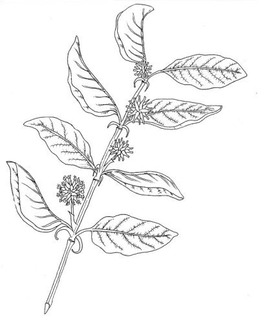
plant morphology
Comparative morphology
Flowers 1.5 to 2 cm, arranged in clusters of capitulum, sessile, glabrous corolla Of 2-3 cm, arranged in clusters of capitulum, stalked, hairy corolla pubescent
Fruit Bivalve capsules, narrowly oblong ovate, up to 9 mm in length Capsules of 2-3 cm without pedicel Habitat Earthy-clay soils, from 0-500 m altitude
It is found in Loreto: Santiago river mouth; San Martin: Mariscal Cáceres; Junin: Chanchamayo, La Merced; Pasco: Oxapampa, Pozuzo; Madre de Dios: Manu, Tahuamanu; Cusco: La Convencion, Paucartambo Earthy-clay soils, from 0-500 m altitude
It is found in Loreto: Yurimaguas, Port Arthur, Itaya River, La Campuya; San Martin: Tarapoto; in Ayacucho: Chocmacota Valley; in Cusco: Cosnipata; Madre de Dios:
- Uncaria tomentosa (Willd.) DC. (1830)
- Uncaria guianensis (Aubl.) Gmel. (1796)
Flowers 1.5 to 2 cm, arranged in clusters of capitulum, sessile, glabrous corolla Of 2-3 cm, arranged in clusters of capitulum, stalked, hairy corolla pubescent
Fruit Bivalve capsules, narrowly oblong ovate, up to 9 mm in length Capsules of 2-3 cm without pedicel Habitat Earthy-clay soils, from 0-500 m altitude
It is found in Loreto: Santiago river mouth; San Martin: Mariscal Cáceres; Junin: Chanchamayo, La Merced; Pasco: Oxapampa, Pozuzo; Madre de Dios: Manu, Tahuamanu; Cusco: La Convencion, Paucartambo Earthy-clay soils, from 0-500 m altitude
It is found in Loreto: Yurimaguas, Port Arthur, Itaya River, La Campuya; San Martin: Tarapoto; in Ayacucho: Chocmacota Valley; in Cusco: Cosnipata; Madre de Dios:
scientific name
- Uncaria tomentosa (Willd.) DC. (1830)
- Uncaria guianensis (Aubl.) J. F. Gmel. (1796)
synonym
1. Uncaria tomentosa (Willd.) DC. (1830) - Nauclea aculeata (HBK) (Nov. Gen & sp 3: 382. 1819 no Willd.) - N. tomentosa Willd. ex R. & S. (Syst Veg 5: 221. 1819) - Orouparia tomentosa (Willd Ex R. & S.) Schumi (Fl Mart Bras 6 PT 6: 132, 1889)
2. Uncaria guianensis (Aubl.) Gmel. (1796) - Oruparia guianensis Aublet in 1775 - Uncaria guianensis Schreber in 1789
2. Uncaria guianensis (Aubl.) Gmel. (1796) - Oruparia guianensis Aublet in 1775 - Uncaria guianensis Schreber in 1789
common names
1. Uncaria tomentosa (Willd.) DC. (1830) - Garabato: (Huallaga) (Forest) Peru - Unganangui: Peruvian Forest - Yellow Doodle: Peruvian Forest - Samento: Ashaninka, Peru - Kug kukjaqui: Aguaruna, Huambisa, Jibaro (Marañón) - Paotati - Mosha: Shipibo-Conibo ethnic group - Misho - mentis: Shipibo-Conibo ethnic group
2. Uncaria guianensis (Aubl.) Gmel. (1796) - Cat's claw - Claw hawk - Garabato Colorado - Unganangui
- Tambor huasca: natives of the stream of Momón River - Iquitos - Garabato casha - Ancayacu - Paraguayan - Ancay sillo
2. Uncaria guianensis (Aubl.) Gmel. (1796) - Cat's claw - Claw hawk - Garabato Colorado - Unganangui
- Tambor huasca: natives of the stream of Momón River - Iquitos - Garabato casha - Ancayacu - Paraguayan - Ancay sillo
ethnomedicine
chemical constituents
Phytochemical investigations on Uncaria tomentosa and U. guianensis revealed the presence of mainly three types of secondary metabolites:
Both species are found in Peru and have long been used as part of traditional medicine.
Amongst the alkaloid compounds, the following have been identified:
Also, some of their corresponding N-oxides and its indole precursors (akuammigine, tetrahydroalstonine, isoajmalicine, hirsutine, dihydrocorynantheine, hirsuteine, and corynantheine) have been reported, as well presence of harmane, 5αcarboxystrictosidine.
The following is noteworthy:
Also, it is important to mention that no significant difference has been found in the ratio of oxindole alkaloids between leaves and roots.
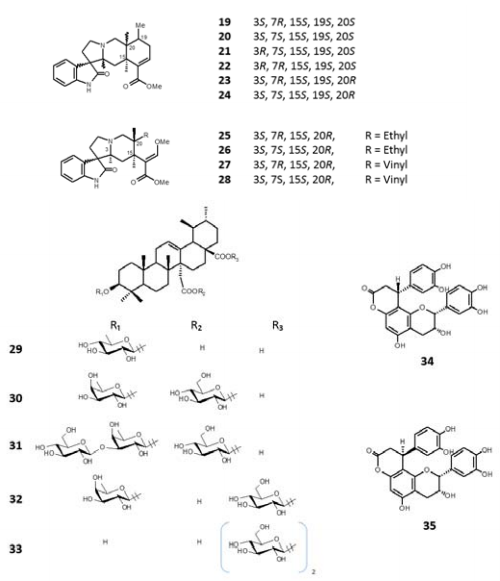
Two groups should be considered in the triterpene compounds: the quinovic acid and its glycosides, and the polyhydroxylated triterpenes.
In the first group, 16 other structures of the glycosylated quinovic acid have been found, for example:
From these, four are common for both species, two have been reported only on U. guianensis, and the other 11 only on U. tomentosa.
On the other hand, the sugar units from glycosides are: glucose, fucose, quinovose, rhamnose, and galactose, which can be in positions C-3, C-27, C-28, C-3, 27 or C-3, 28. These sugar units are repeated 16 times in glucose, 7 in fucose, 4 in quinovose, 3 in rhamnose, and 1 in galactose.
- indole and
- oxindole alkaloids, triterpenes as quinovic acid glycosides and
- polyphenols.
Both species are found in Peru and have long been used as part of traditional medicine.
Amongst the alkaloid compounds, the following have been identified:
- pentacyclic oxindole,
- AOP (pterodine, isopteropodine, speciophylline, uncarine F, mitraphylline, and isomitraphylline: 19- 24, respectively); and tetracyclic,
- AOT (rhynchophylline, isorhynchophylline, corynoxeine, isocorynoxeine: 25-28, respectively).
Also, some of their corresponding N-oxides and its indole precursors (akuammigine, tetrahydroalstonine, isoajmalicine, hirsutine, dihydrocorynantheine, hirsuteine, and corynantheine) have been reported, as well presence of harmane, 5αcarboxystrictosidine.
The following is noteworthy:
- (a) the concentration of alkaloids is lower in the U. guianensis and that some of them have not been found in this species, maybe due to the lack of sufficient studies;
- (b) different samples analyzed show variation of the total content of alkaloids, as well as the individual alkaloids. In addition, it has been determined that there are two chemical-types for the U. tomentosa, one that mainly (or only) contains AOP and a second one with AOT.
Also, it is important to mention that no significant difference has been found in the ratio of oxindole alkaloids between leaves and roots.
Two groups should be considered in the triterpene compounds: the quinovic acid and its glycosides, and the polyhydroxylated triterpenes.
In the first group, 16 other structures of the glycosylated quinovic acid have been found, for example:
- quinovic acid 3β-O-βD- quinovopyranoside,
- quinovic acid 3β-O-β-D-fucopyranosyl- (27→1)-D-glucopyranoylester,
- quinovic acid 3β-O-[β-Dglucopyranosil-(1→3)-β-D-fucopyranosyl]-(27→1)
- β-Dglucopyranosylester (29-31).
From these, four are common for both species, two have been reported only on U. guianensis, and the other 11 only on U. tomentosa.
On the other hand, the sugar units from glycosides are: glucose, fucose, quinovose, rhamnose, and galactose, which can be in positions C-3, C-27, C-28, C-3, 27 or C-3, 28. These sugar units are repeated 16 times in glucose, 7 in fucose, 4 in quinovose, 3 in rhamnose, and 1 in galactose.
Other polyhydroxylated triterpenes from U. tomentosa have been isolated.
These are derived from ursolic acid, quinovic acid, and glycosides of nortriterpenes.
Also, other compounds isolated include triterpenes: lupeol, ursolic and oleanolic acids, and sterols like β-sitosterol, campesterol and stigmasterol.
Likewise, the presence of the iridoid, 7- deoxyloganic acid has been described.
Some phenolic compounds were reported as cinchonains Ia and Ib (34, 35) and quinic acid derivatives, amongst these, 3, 4-Odicaffeoylquinic acid, 3-O-feruloylquinic acid and the 3-Ocaffeoylquinic acid, known as carboxylalkyl esters (Figure 3).
These are derived from ursolic acid, quinovic acid, and glycosides of nortriterpenes.
Also, other compounds isolated include triterpenes: lupeol, ursolic and oleanolic acids, and sterols like β-sitosterol, campesterol and stigmasterol.
Likewise, the presence of the iridoid, 7- deoxyloganic acid has been described.
Some phenolic compounds were reported as cinchonains Ia and Ib (34, 35) and quinic acid derivatives, amongst these, 3, 4-Odicaffeoylquinic acid, 3-O-feruloylquinic acid and the 3-Ocaffeoylquinic acid, known as carboxylalkyl esters (Figure 3).
Figure 3: Some chemical compounds isolated from Uncaria tomentosa/U.guianesis.
pharmacology
Ethanolic and aqueous extracts of Uncaria tomentosa (UT) showed promising antioxidant activities measured through TEAC (Trolox equivalent antioxidant capacity), PRTC (Peroxyl radical-trapping capacity) and SOD (Superoxide radical scavenging activity) tests.
High antioxidant activities were also detected in DPPH, SOD and PRTC tests and by inhibition of lipid peroxidation.
According to Gonçalves et al, these activities could be explained by the high content of proanthocyanidins and phenolic acids, mainly caffeic acid, in the extract.
Methanol extracts of stem-bark and roots of UT showed antioxidant activity in rat liver homogenates, by preventing thiobarbituric acid reactive substances production and free radical-mediated DNAsugar damage.
Antimicrobial properties have been reported for cat´s claw. Ethanol bark extracts of Uncaria guianensis (UG) was active against multidrug-resistant Staphylococcus aureus and Pseudomonas aeruginosa.
UT showed activity against oral human pathogens Streptococcus mutans, Staphylococcus spp. and Enterobacteriaceae.
A bioassay-guided fractionation of UT led to the isolation of isopteropodine as an antibacterial active principle against Gram positive bacteria.
Pheophorbide A ethyl ester was isolated from UG leaves. This compound showed antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli and Salmonella typhimurium.
Caon et al. demonstrated an antiherpetic activity for the hydroethanolic extract from bark of UT. However, purified fractions of quinovic acid glycosides and oxindole alkaloids were less active, suggesting a synergetic effect. The probable mechanism of action is the inhibition of viral attachment in the host cell.
Pentacyclic oxindole alkaloid-enriched fraction was the most effective in reducing monocyte infection with dengue virus-2 (DENV-2).
This same alkaloidal fraction showed antiviral activity, as well as reduction of endotelial permeability, on human dermal microvascular endotelial cells infected with DENV-2.
Riva et al. found that UT bark extracts and fractions exert an antiproliferative activity on MCF7.
UT extracts, with different concentrations of alkaloid contents had antiproliferative effects on HL-60 acute promyelocytic human cells.
Cytotoxic activities of UT extracts have been related to the alkaloids. Uncarine D exhibited weak cytotoxic activity against SKMEL, KB, BT-549 and SK-OV-3 cell lines, while uncarine C showed low cytotoxicity only against ovarian carcinoma.
Mitraphylline inhibited the growth of human Ewing's sarcoma MHHES1 and breast cancer MT-3 cell lines, with IC50 values of 17.15 ± 0.82 and 11.80 ± 1.03 μM, respectively. Both IC50 values were smaller than those obtained for the reference compounds cyclophosphamide and vincristine.
Micromolar concentrations of mitraphylline inhibited the growth of glioma and neuroblastoma cell lines.
Pteropodine exhibited good antioxidant activity in the DPPH test, increased the production of lymphocytes and decreased bone marrow cytotoxicity induced by doxorubicin.
Pteropodine and uncarine F induce apoptosis on acute leukaemic lymphoblasts.
UT has also anticancer activity in vivo. A hydroethanolic extract of UT inhibited B16/BL6 melanoma cell growth and metastasis in mice. UT decreased TNF-α, IL-6 and NO production in vitro. NFκB activity was also inhibited in LPS-stimulated HeLa cells.
Dreifuss et al. suggested that the anticancer activity in vivo (Walker-256 tumour) shown by UT extracts may be a result of a synergic combination of substances, most of them antioxidant compounds.
C-Med-100®, an aqueous extract of UT, inhibits the growth of HL60 and Raji cells by producing DNA strand breaks coupled to selective apoptosis.
However, in an in vivo model, the extract inhibits proliferation of normal mouse T and B lymphocytes; this inhibition was not caused by induction of apoptosis.
According to Åkesson et al., the extract induces cell proliferation arrest and inhibits activation of the transcriptional regulator (NF-κB in vitro. This effect may be due to the presence of quinic acid in the extract.
High antioxidant activities were also detected in DPPH, SOD and PRTC tests and by inhibition of lipid peroxidation.
According to Gonçalves et al, these activities could be explained by the high content of proanthocyanidins and phenolic acids, mainly caffeic acid, in the extract.
Methanol extracts of stem-bark and roots of UT showed antioxidant activity in rat liver homogenates, by preventing thiobarbituric acid reactive substances production and free radical-mediated DNAsugar damage.
Antimicrobial properties have been reported for cat´s claw. Ethanol bark extracts of Uncaria guianensis (UG) was active against multidrug-resistant Staphylococcus aureus and Pseudomonas aeruginosa.
UT showed activity against oral human pathogens Streptococcus mutans, Staphylococcus spp. and Enterobacteriaceae.
A bioassay-guided fractionation of UT led to the isolation of isopteropodine as an antibacterial active principle against Gram positive bacteria.
Pheophorbide A ethyl ester was isolated from UG leaves. This compound showed antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli and Salmonella typhimurium.
Caon et al. demonstrated an antiherpetic activity for the hydroethanolic extract from bark of UT. However, purified fractions of quinovic acid glycosides and oxindole alkaloids were less active, suggesting a synergetic effect. The probable mechanism of action is the inhibition of viral attachment in the host cell.
Pentacyclic oxindole alkaloid-enriched fraction was the most effective in reducing monocyte infection with dengue virus-2 (DENV-2).
This same alkaloidal fraction showed antiviral activity, as well as reduction of endotelial permeability, on human dermal microvascular endotelial cells infected with DENV-2.
Riva et al. found that UT bark extracts and fractions exert an antiproliferative activity on MCF7.
UT extracts, with different concentrations of alkaloid contents had antiproliferative effects on HL-60 acute promyelocytic human cells.
Cytotoxic activities of UT extracts have been related to the alkaloids. Uncarine D exhibited weak cytotoxic activity against SKMEL, KB, BT-549 and SK-OV-3 cell lines, while uncarine C showed low cytotoxicity only against ovarian carcinoma.
Mitraphylline inhibited the growth of human Ewing's sarcoma MHHES1 and breast cancer MT-3 cell lines, with IC50 values of 17.15 ± 0.82 and 11.80 ± 1.03 μM, respectively. Both IC50 values were smaller than those obtained for the reference compounds cyclophosphamide and vincristine.
Micromolar concentrations of mitraphylline inhibited the growth of glioma and neuroblastoma cell lines.
Pteropodine exhibited good antioxidant activity in the DPPH test, increased the production of lymphocytes and decreased bone marrow cytotoxicity induced by doxorubicin.
Pteropodine and uncarine F induce apoptosis on acute leukaemic lymphoblasts.
UT has also anticancer activity in vivo. A hydroethanolic extract of UT inhibited B16/BL6 melanoma cell growth and metastasis in mice. UT decreased TNF-α, IL-6 and NO production in vitro. NFκB activity was also inhibited in LPS-stimulated HeLa cells.
Dreifuss et al. suggested that the anticancer activity in vivo (Walker-256 tumour) shown by UT extracts may be a result of a synergic combination of substances, most of them antioxidant compounds.
C-Med-100®, an aqueous extract of UT, inhibits the growth of HL60 and Raji cells by producing DNA strand breaks coupled to selective apoptosis.
However, in an in vivo model, the extract inhibits proliferation of normal mouse T and B lymphocytes; this inhibition was not caused by induction of apoptosis.
According to Åkesson et al., the extract induces cell proliferation arrest and inhibits activation of the transcriptional regulator (NF-κB in vitro. This effect may be due to the presence of quinic acid in the extract.
Co-incubation with C-Med-100 with skin cell protected them from UV exposure; this protection occurred with a concomitant increase in DNA repair.
The hydroalcoholic extract of UT showed anti-inflammatory activity in the carrageenan-induced paw edema model in mice. It also showed little inhibitory activity on cyclooxygenase-1 and -2.
According to Aquino et al, a bioassay-directed fractionation of UT extract showed that one of the active antiinflammatory principles is a quinovic acid glycoside.
Oral pretreatment of mice with an ethanolic extract of UG leaves decreased paw oedema and pleural exudation induced by zymosan or ovoalbumin.
A subfraction of a hydroethanolic extract of UG bark inhibited NO, TNF-α, IL-6 and PGE2 production by macrophages in vitro and in the serum of LPS-challenged mice. Macrophage expression of IκB degradation was completely inhibited, while NF-κB activation was inhibited by 70%. UG subfraction also decreased serum NO, TNFα, paw oedema induced by carrageenan and mammary tumour growth by 91%.
Wagner et al. reported that four out of six oxindole alkaloids present UT caused a pronounced enhancement of phagocytosis, both in vitro and in vivo [68d]. UG and UT showed antioxidant activity (DPPH test) and strong ability to inhibit TNF-α production in RAW 264.7 cells.
In THP-1 cells, UT also decreases TNF-α and has a opposite effect on IL-1β and IL-6.
UG was more active than UT in scavenging DPPH and hydroxyl radicals, and in inhibiting lipid peroxidation. The inhibition of TNFα production was significantly higher for UT. Non-alkaloid HPLC fractions from UT decreased LPS-induced TNF-α production. Thus, the presence of oxindolic alkaloids did not influence the antioxidant and antiinflammatory properties of UT. Oral pretreatment with UT protected against indomethacin-induced gastritis in rats.
In mice subjected to bacterial lipopolysaccharide endotoxin, Mitraphylline inhibited around 50% of the release of interleukins 1α, 1β, 4, 17 and TNF-α. This activity was very similar to that of dexamethasone.
UG extract decreased peroxynitrite-induced apoptosis in HT29 and RAW 264.7 cells. It also inhibited lipopolysaccharide-induced iNOS gene expression, nitrite formation, cell death and inhibited the activation of NF-κB. Moreover, it attenuated indomethacin-enteritis.
According to Allen-Hall et al., UT inhibits NF-κB pathway activation, leading to a decrease in TNF-production and low cell proliferation. Thus, UT has a potential therapeutic use as anticancer or anti-inflammatory agent.
An alkaloid-enriched preparation from UT inhibits NF-κB in promyelocytic leukemia HL-60 cells. Pentacyclic oxindole alkaloids may be the active compounds responsible for this effect.
The hydroalcoholic extract of UT showed anti-inflammatory activity in the carrageenan-induced paw edema model in mice. It also showed little inhibitory activity on cyclooxygenase-1 and -2.
According to Aquino et al, a bioassay-directed fractionation of UT extract showed that one of the active antiinflammatory principles is a quinovic acid glycoside.
Oral pretreatment of mice with an ethanolic extract of UG leaves decreased paw oedema and pleural exudation induced by zymosan or ovoalbumin.
A subfraction of a hydroethanolic extract of UG bark inhibited NO, TNF-α, IL-6 and PGE2 production by macrophages in vitro and in the serum of LPS-challenged mice. Macrophage expression of IκB degradation was completely inhibited, while NF-κB activation was inhibited by 70%. UG subfraction also decreased serum NO, TNFα, paw oedema induced by carrageenan and mammary tumour growth by 91%.
Wagner et al. reported that four out of six oxindole alkaloids present UT caused a pronounced enhancement of phagocytosis, both in vitro and in vivo [68d]. UG and UT showed antioxidant activity (DPPH test) and strong ability to inhibit TNF-α production in RAW 264.7 cells.
In THP-1 cells, UT also decreases TNF-α and has a opposite effect on IL-1β and IL-6.
UG was more active than UT in scavenging DPPH and hydroxyl radicals, and in inhibiting lipid peroxidation. The inhibition of TNFα production was significantly higher for UT. Non-alkaloid HPLC fractions from UT decreased LPS-induced TNF-α production. Thus, the presence of oxindolic alkaloids did not influence the antioxidant and antiinflammatory properties of UT. Oral pretreatment with UT protected against indomethacin-induced gastritis in rats.
In mice subjected to bacterial lipopolysaccharide endotoxin, Mitraphylline inhibited around 50% of the release of interleukins 1α, 1β, 4, 17 and TNF-α. This activity was very similar to that of dexamethasone.
UG extract decreased peroxynitrite-induced apoptosis in HT29 and RAW 264.7 cells. It also inhibited lipopolysaccharide-induced iNOS gene expression, nitrite formation, cell death and inhibited the activation of NF-κB. Moreover, it attenuated indomethacin-enteritis.
According to Allen-Hall et al., UT inhibits NF-κB pathway activation, leading to a decrease in TNF-production and low cell proliferation. Thus, UT has a potential therapeutic use as anticancer or anti-inflammatory agent.
An alkaloid-enriched preparation from UT inhibits NF-κB in promyelocytic leukemia HL-60 cells. Pentacyclic oxindole alkaloids may be the active compounds responsible for this effect.
Quinovic acid glycosides purified fraction of U. tomentosa decreased the growth and viability of human bladder cancer cell lines by inducing apoptosis through modulation of NF-κB.
Quinic acid enhances DNA repair and has a neuroprotective effect in neurons. It can improve Caenorhabidits elegans survival under oxidative stress by upregulating the expression of the small heat shock protein hsp-16.2 gene. Quinic acid may have potential as a rejuvenating agent.
According to Uchida et al., UG slightly inhibited the progression of the atherosclerosis in Watanabe heritable hyperlipidemic rabbits. UG inhibited oxidation of LDL, decreased total cholesterol, triglycerides, and the percent of plaque area formation.
Quinic acid enhances DNA repair and has a neuroprotective effect in neurons. It can improve Caenorhabidits elegans survival under oxidative stress by upregulating the expression of the small heat shock protein hsp-16.2 gene. Quinic acid may have potential as a rejuvenating agent.
According to Uchida et al., UG slightly inhibited the progression of the atherosclerosis in Watanabe heritable hyperlipidemic rabbits. UG inhibited oxidation of LDL, decreased total cholesterol, triglycerides, and the percent of plaque area formation.
clinical aspects
Concerning the antiinflammatory activity, there are three clinical studies which evaluated the effect of U. tomentosa (Willd) DC; in osteoarthritis and in rheumatoid arthritis.
The quality of these studies was evaluated as poor, according to Natural Standard database; however, they performed well on reducing pain, improving joint range and decreasing PGE2 and TNF- levels.
The conclusions were that both uncarias are effective for the treatment of osteoarthritis; these results are reinforced by the systematic review prepared by Rosenbaum.
In 2008, a clinical trial conducted in patients with rheumatoid arthritis showed that the treatment with U. tomentosa (Willd) inhibits the activation of NF-kB, the expression of COX enzyme and accelerates the maturation/activation of the subpopulation of dendritic cells.
All these mechanisms are important in the pathogenesis of rheumatoid arthritis, so it is justified to continue with larger trials in order to verify UT efficacy. Natural Standard evaluated a clinical study to verify the immuno-stimulatory activity of UT.
This study was classified as poor quality; however, it was able to demonstrate the stimulating effect on the immune system.
All these studies suggest that U. tomentosa is able to boost immune function.
The quality of these studies was evaluated as poor, according to Natural Standard database; however, they performed well on reducing pain, improving joint range and decreasing PGE2 and TNF- levels.
The conclusions were that both uncarias are effective for the treatment of osteoarthritis; these results are reinforced by the systematic review prepared by Rosenbaum.
In 2008, a clinical trial conducted in patients with rheumatoid arthritis showed that the treatment with U. tomentosa (Willd) inhibits the activation of NF-kB, the expression of COX enzyme and accelerates the maturation/activation of the subpopulation of dendritic cells.
All these mechanisms are important in the pathogenesis of rheumatoid arthritis, so it is justified to continue with larger trials in order to verify UT efficacy. Natural Standard evaluated a clinical study to verify the immuno-stimulatory activity of UT.
This study was classified as poor quality; however, it was able to demonstrate the stimulating effect on the immune system.
All these studies suggest that U. tomentosa is able to boost immune function.
econimic importance
The two known species of cat’s claw are used traditionally. They are commonly found in supplements and have numerous medicinal demands. Extracts of cat’s claw bark are utilized mainly as dietary supplements for supporting or improving immune system functions, as well as in medicinal products for arthritic conditions, and to a lesser extent in liquid preparations for topical application, sometimes in combination with other Andean botanicals such as dragon’s blood croton (Croton lechleri Muell. Arg.); both of them are regionally and globally marketed.
According to the United States Pharmacopeia, cat's claw consists of the inner bark of the stems of Uncaria tomentosa which contains no less than 0.3 percent of pentacyclic oxindole alkaloids, calculated on the dried basis, as the sum of:
On the other hand ITC listed the most important medicinal and aromatic plants that are produced in one or more South American countries, some of them are:
Cat’s claw has been promoted under national policies and has a significant international market.
Patents on the chemicals derived from cat’s claw (UT) failed to acknowledge or compensate the source countries and indigenous cultures that held the knowledge of the plants’ healing qualities.
At the moment, there are 555 applications detected by National Biopiracy Commision.
Peru prohibits exports of certain specimens of Cat's Claw (Uncaria tomentosa and Uncaria guianensis) that are "either unprocessed or subject to mechanical processing", unless they come from specific areas.
According to the United States Pharmacopeia, cat's claw consists of the inner bark of the stems of Uncaria tomentosa which contains no less than 0.3 percent of pentacyclic oxindole alkaloids, calculated on the dried basis, as the sum of:
- speciophylline,
- uncarine F,
- mitraphylline,
- isomitraphylline,
- pteropodine and
- isopteropodine.
On the other hand ITC listed the most important medicinal and aromatic plants that are produced in one or more South American countries, some of them are:
- a) Uncaria tomentosa: standardized botanical extract (1.0-1.5% total alkaloids by HPLC) from Brazil.
- b) Uncaria tomentosa: standardized botanical extract (2% total alcaloids by HPLC) from Peru.
Cat’s claw has been promoted under national policies and has a significant international market.
Patents on the chemicals derived from cat’s claw (UT) failed to acknowledge or compensate the source countries and indigenous cultures that held the knowledge of the plants’ healing qualities.
At the moment, there are 555 applications detected by National Biopiracy Commision.
Peru prohibits exports of certain specimens of Cat's Claw (Uncaria tomentosa and Uncaria guianensis) that are "either unprocessed or subject to mechanical processing", unless they come from specific areas.
mitraphylline
A pentacyclic oxindolic alkaloid that was isolated from the alkaloid fraction of the dried inner bark of Uncaria tomentosa (Willd. ex Schult.) DC; it represents the most abundant phytochemical (40%) of the alkaloid fraction.
Several investigations have demonstrated the immunoregulatory activity of this compound or the pentacyclic oxindolic alkaloid-enriched fraction.
The mechanism of action as immunoregulator of mitraphylline consists in both to protect cells against oxidative stress and to elicit a response via an NF-kβ-dependent mechanism. The first mechanism is based on the inhibition of the inducible nitric oxide synthase gene expression; consequently, nitrite formation and programmed cell death are avoided. Finally, in the second mechanism, the inhibition of NF-kβ signaling permits the abrogation of the release of pro-inflammatory cytokines such as TNFα, IL-6, IL-1 α, IL-1β, IL-4, IL-17, and IFN-α.
Several investigations have demonstrated the immunoregulatory activity of this compound or the pentacyclic oxindolic alkaloid-enriched fraction.
The mechanism of action as immunoregulator of mitraphylline consists in both to protect cells against oxidative stress and to elicit a response via an NF-kβ-dependent mechanism. The first mechanism is based on the inhibition of the inducible nitric oxide synthase gene expression; consequently, nitrite formation and programmed cell death are avoided. Finally, in the second mechanism, the inhibition of NF-kβ signaling permits the abrogation of the release of pro-inflammatory cytokines such as TNFα, IL-6, IL-1 α, IL-1β, IL-4, IL-17, and IFN-α.