|
ŠTO JE AGUAJE?
Aguaje je plod koji raste na diolnom stablu, odnosno njegova vrsta ima muško i žensko stablo, ovo voće raste samo na ženskim stablima. Konzumiranje aguaje ima iznenađujuća svojstva kod žena koje ih konzumiraju zbog velike količine fitoestrogena koje posjeduje, zbog čega mnogi pripisuju aguaje ljepoti i raskoši žena peruanske džungle, koje ga redovno konzumiraju. |
WHAT IS AGUAJE?
Aguaje is a fruit that grows on a diol tree, that is, its species has both a male and a female tree, this fruit grows only on female trees. Consuming aguaya has surprising properties in women who consume it because of the large amount of phytoestrogens it possesses, which is why many attribute it to the beauty and luxuries of Peruvian jungle women, who regularly consume it. |
|
POMAŽE U UČINKOVITOM OBLIKOVANJU TIJELA:
To uključuje povećanje grudi, smanjivanje gluteusa i struka; Zbog svog visokog sadržaja fitoestrogena, koji su "biljni hormoni", oni ispunjavaju funkciju harmonizacije ljudskih hormona i, zauzvrat, proporcija u tijelu, bez posljedica sintetskih ili životinjskih hormona, koji mijenjaju organizam |
HELPS TO SHAPE YOUR BODY EFFECTIVELY
These include breast enlargement, gluteus and waist reduction; Due to their high content of phytoestrogens, which are "plant hormones", they fulfill the function of harmonizing human hormones and, in turn, the proportion in the body, without the consequences of synthetic or animal hormones, that alter the body |
|
ODLIČAN HORMONALNI REGULATOR
Zbog svog estrogenog djelovanja (koji na najprikladniji način stimulira estrogene), njegova se upotreba širila za ublažavanje simptoma povezanih s hormonskim poremećajima (poput menstrualne boli, neplodnosti, promjene raspoloženja, vrućih bljeskova i dr. proizvodnja prolaktina, uključujući sve one povezane s menopauzom, da spomenemo najvažnije). Nakon brojnih istraživanja utvrđeno je da se prehrambeno ponašanje (kineske) populacije koja tradicionalno konzumira fitoestrogene pretvara u kasnu menopauzu i manje poremećaje povezane s hormonima. Dakle, bilo koje stanje povezano s tim hormonima je regulirano. |
EXCELLENT HORMONAL REGULATOR
Due to its estrogenic action (which most appropriately stimulates estrogens), its use has spread to alleviate symptoms associated with hormonal disorders (such as menstrual pain, infertility, mood swings, hot flashes, etc.) Prolactin production, including all menopause-related ones mention the most important). Numerous studies have shown that the dietary behavior of the (Chinese) population that traditionally consumes phytoestrogens translates into late menopause and minor hormone-related disorders. So any condition associated with these hormones is regulated. |
|
ANTI-TUMORSKA SVOJSTVA
Fitoestrogeni imaju antikancerogeni učinak, jer hormoni koji se reguliraju ne dopuštaju stvaranje cista ili "super produkciju" stanica, što uzrokuje tumore ili miome. |
ANTI-TUMOR PROPERTIES
Phytoestrogens have an anticancer effect because the hormones that are regulated do not allow cyst formation or "super-production" of cells, which causes tumors or myomas. |
|
SNAŽAN ANTIOKSIDANS
Zbog visokog sadržaja alfatokoferola učvršćuje kožu, čini je blistavom , a zauzvrat pomaže poboljšati izgled kose i noktiju. |
A POWERFUL ANTIOXIDANT
Due to its high content of alphatocopherol, it tightens the skin, makes it radiant, and in turn helps to improve the appearance of hair and nails. |
|
FITOESTROGENI
Fitoestrogeni su kemijski spojevi koji se nalaze u nekom povrću s aktivnostima sličnim ljudskim estrogenima, tako da mogu pomoći metabolizmu masti smanjujući razinu tijela i pogoduju sintezi kalcija i koštanog sustava. Voće poput aguaje bogato je fitoestrogenima i vitaminima. |
PHYTOESTROGENS
Phytoestrogens are chemical compounds found in some vegetables with activities similar to human estrogens, so they can help metabolize fat by reducing body levels and favor the synthesis of calcium and the bone system. Fruits like aguayas are rich in phytoestrogens and vitamins. |
|
SITOSTEROL
To je kemijski spoj koji pripada skupini fitosterola, a to su steroli koji se prirodno nalaze u biljkama. Kemijska je struktura vrlo slična onoj kolesterola. Rasprostranjena je u biljnom svijetu gdje ispunjavaju funkciju održavanja strukture i funkcioniranja staničnih membrana. |
SITOSTEROL
It is a chemical compound belonging to the phytosterol group, and these are sterols naturally found in plants. The chemical structure is very similar to that of cholesterol. It is widespread in the plant world where they fulfill the function of maintaining the structure and function of cell membranes. |
|
VITAMIN A
Vitamin A, retinol ili antikseroftalmik je vitamin topiv u mastima (to jest, topiv je u masnim tijelima, uljima i ne može se izbaciti u urinu kao što to obično čine vitamini topljivi u vodi) koji sudjeluju u stvaranju i održavanju stanica epitela, u rastu kostiju, razvoju, zaštiti i regulaciji kože i sluznice. Vitamin A je neophodan hranjivi sastojak za ljude. Poznat je i kao retinol, jer stvara pigmente potrebne za rad mrežnice. On igra važnu ulogu u razvoju dobrog vida, posebno pri slabom svjetlu. Također se može koristiti za razmnožavanje i dojenje. Β-karoten, koji ima antioksidacijska svojstva koja pomažu u uklanjanju slobodnih radikala koji sprečavaju starenje stanica, Prekursor je vitamina A. Retinol može oksidirati i tvori retinoičnu kiselinu, kiselinu za medicinsku upotrebu. Ovaj vitamin ima 3 vitamina (vitamini koji imaju više od jednog kemijskog oblika) su retinol, mrežnica i retinoična kiselina. Nastaje iz provitamina beta karotena i drugih provitamina u traktu debelog crijeva. Čuva se u jetri. |
VITAMIN A
Vitamin A, retinol or antixerophthalmic is a fat soluble vitamin (that is, it is soluble in fat bodies, oils, and cannot be excreted in the urine as is usually water-soluble vitamins) that are involved in the production and maintenance of epithelial cells, in growth bone, the development, protection and regulation of the skin and mucous membranes. Vitamin A is a necessary nutrient for humans. Also known as retinol, it creates the pigments needed for the retina to function. It plays an important role in the development of good vision, especially in low light. It can also be used for reproduction and breastfeeding. Β-Carotene, which has antioxidant properties that help eliminate free radicals that prevent cell aging, is a precursor of Vitamin A. Retinol can oxidize and form retinoic acid, an acid for medical use. This vitamin has 3 vitamins (vitamins that have more than one chemical form) are retinol, retina and retinoic acid. It is formed from the provitamins of beta-carotene and other provitamins in the colon. It is stored in the liver. |
|
VITAMIN C
Vitamin C, enantiomer L askorbinske ili antikorbutinske kiseline, ključan je hranjivi sastojak, posebno za sisavce. Prisutnost ovog vitamina potrebna je za određeni broj metaboličkih reakcija kod svih životinja i biljaka, a interno ih stvaraju gotovo svi organizmi, s tim da su ljudi značajna iznimka. Njegov nedostatak uzrokuje skorb kod ljudi, otuda i naziv askorbinska koja se daje kiselini, pa se široko koristi kao aditiv u hrani za sprečavanje njezina nastanka. Farmakofor vitamina C je ion askorbata. U živim organizmima askorbat je antioksidans, jer štiti tijelo od oksidacije, a kofaktor je u nekoliko vitalnih enzimskih reakcija. Svakodnevne potrebe i potrebe ovog vitamina izvor su rasprave. Tvrdi se da su ljudi koji jedu dijetu bogatu askorbinskom kiselinom iz prirodnih izvora, poput voća i povrća, zdraviji i imaju nižu smrtnost i manje kroničnih bolesti. Međutim, metaanaliza 68 pouzdanih pokusa u kojima je korištena suplementacija vitamina C, a koja je uključivala 232.606 pojedinaca, zaključila je da dodatna konzumacija askorbata kroz suplemente možda neće biti korisna kao što se prije mislilo. |
VITAMIN C
Vitamin C, the enantiomer of L ascorbic or anticorbutic acid, is a key nutrient, especially for mammals. The presence of this vitamin is required for a number of metabolic reactions in all animals and plants, and is internally generated by almost all organisms, with humans being a significant exception. Its deficiency causes scurvy in humans, hence the name ascorbic, which is given to acid, so it is widely used as an additive in food to prevent its occurrence. The pharmacophore of vitamin C is an ascorbate ion. In living organisms, ascorbate is an antioxidant because it protects the body from oxidation, and cofactor is in several vital enzymatic reactions. The daily needs and needs of this vitamin are a source of debate. It is claimed that people who eat a diet rich in ascorbic acid from natural sources, such as fruits and vegetables, are healthier and have lower mortality and fewer chronic diseases. However, a meta-analysis of 68 reliable trials using vitamin C supplementation involving 232,606 individuals concluded that additional ascorbate consumption through supplements may not be as useful as previously thought. |
|
VITAMIN E
Α-tokoferol ili vitamin E je vitamin topiv u mastima koji djeluje kao antioksidans na nivou membrane u stanicama (citoplazmatski, lizosomski, endoplazmatski retikulum itd.). |
VITAMIN E
Α-Tocopherol or Vitamin E is a fat soluble vitamin that acts as a membrane-level antioxidant in cells (cytoplasmic, lysosomal, endoplasmic reticulum, etc.). |
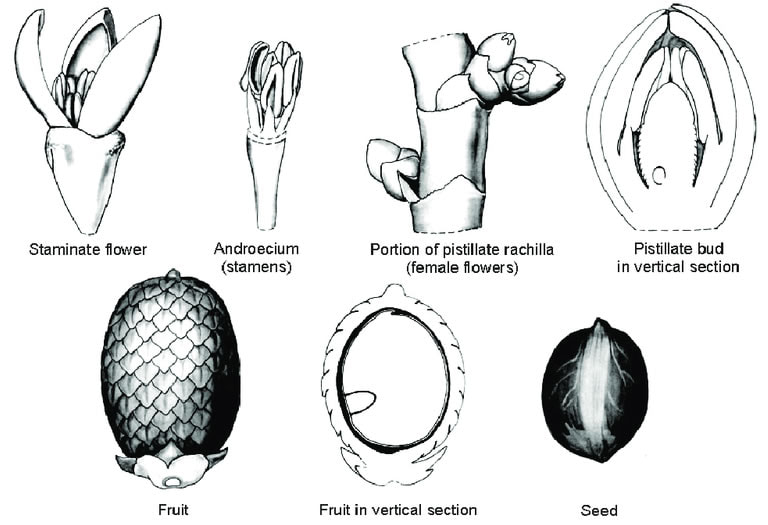
Mauritia flexuosa
|
Poznata kao palma moriche, ité palma, ita, buriti, muriti, canangucho (Kolumbija) ili aguaje (Peru), je palma. Raste u i u blizini močvara i drugih vlažnih područja u tropskoj Južnoj Americi. To je izviješteno iz Trinidada, Kolumbije, Venezuele, Gvajane, Surinama, Francuske Gvajane, Brazila, Ekvadora, Perua i Bolivije.
M. flexuosa, stablo, može doseći do 35 m visine. Veliki listovi tvore zaobljenu krunu. Cvjetovi su žućkasti i pojavljuju se od prosinca do travnja. Plod, koji raste od prosinca do lipnja, kestenjaste je boje i prekriven je sjajnim ljuskama. Žuto meso prekriva tvrdi ovalni orah. Sjemenke lebde, a to je sredstvo kojim se palma razmnožava. U prirodnim populacijama drvo doseže vrlo velike gustoće. |
Known as the moriche palm, ité palm, ita, buriti, muriti, canangucho (Colombia), or aguaje (Peru), is a palm tree. It grows in and near swamps and other wet areas in tropical South America. It has been reported from Trinidad, Colombia, Venezuela, Guyana, Suriname, French Guiana, Brazil, Ecuador, Peru, and Bolivia.
M. flexuosa, a tree, can reach up to 35 m (115 ft) in height. The large leaves form a rounded crown. The flowers are yellowish and appear from December to April. The fruit, which grows from December to June, is a chestnut color and is covered with shiny scales. The yellow flesh covers a hard, oval nut. The seeds float, and this is the means by which the palm tree propagates. In natural populations, the tree reaches very high densities. |
|
Voće palminog voća Moriche jestivo je i koristi se za pravljenje soka, džema, sladoleda, fermentiranog "vina", deserta i grickalica, za koje se u Peruu treba ubrati više od 50 tona dnevno.
|
Fruit Moriche palm fruit is edible and used to make juice, jam, ice cream, a fermented "wine", desserts and snacks, requiring harvesting of more than 50 tonnes per day in Peru.
|
|
Pupovi se jedu kao povrće, a sok se može piti svjež ili fermentiran (vidi palmino vino).
Namještaj i trake lokalno se proizvode od vlakana stabala . Ljudi konzumiraju palmine izdanke koji se ukopavaju u deblo drveća. |
The inflorescence buds are eaten as a vegetable and the sap can be drunk fresh or fermented (see palm wine). Threads and cords are locally produced from the tree's fibers.
Humans consume palm weevils which burrow in the tree trunk. |
|
ULJE M. FLEXUOSE
Buriti ulje je narančasto-crvenkasto ulje izvađeno iz plodova palme moriche. Ulje sadrži visoke koncentracije oleinske kiseline, tokoferole i karotenoide, posebno beta-karoten. Ulje ima crvenkastu boju koja se koristi kao tinta na kožama i koži. |
M. FLEXUOSA OIL
Buriti oil is an orange-reddish oil extracted from the fruit of the moriche palm. The oil contains high concentrations of oleic acid, tocopherols, and carotenoids, especially beta-carotene. The oil has a reddish color used as ink on hides and skins. |
|
EKOLOGIJA
Ovo je drvo važno za mnoge životinjske vrste; nekoliko vrsta ptica, koriste ga za gniježđenje i hranu. Tapiri, ptice, ribe i majmuni ovise o plodu. |
ECOLOGY
This tree is important to many animal species; several bird species, such as the red-bellied macaw, sulphury flycatcher, and moriche oriole, use it for nesting and food. Tapirs, peccaries, fish and monkeys depend on the fruit. |
|
Alexander von Humboldt dokumentirao je stablo i ekosustav koji podržava 1800. godine, putujući kroz područje Llanos u Venezueli. "Sa zaprepašćenjem je promatrao koliko je stvari povezano s postojanjem jedne biljke." Nazvao ga je "stablom života" i u osnovi ga opisao kao ključnu vrstu iako taj pojam eksplicitno ne bi definirao do 1969. Robert T. Paine.
|
Alexander von Humboldt documented the tree and the ecosystem it supports in 1800 when traveling through the Llanos region of Venezuela. He "observed with astonishment how many things are connected with the existence of a single plant." He called it the "tree of life" and essentially described it as a keystone species although the concept would not be explicitly defined until 1969 by Robert T. Paine.
|
terapija protiv trombocita
Podaci ovdje prikazani po prvi put pokazuju zaštitni učinak ekstrakta ulja iz Mauritia flexuosa na aktivaciju trombocita i stvaranje tromboze. Ovi učinci, u smislu primarne prevencije, mogu izmijeniti rizik od kardiovaskularnog sustava bez ikakvih nuspojava koje su obično povezane s lijekovima protiv trombocita. Štoviše, Mauritia flexuosa može predstavljati funkcionalni sastojak koji dodaje aktivne trombocite prerađenoj hrani.
Sažetak za liječnike
Jecko Thachil , savjetnik hematologa A
Podaci o autoru Autorska prava i licenca Izjava o odricanju odgovornosti
Ovaj su članak citirali drugi članci u PMC-u.
Jecko Thachil , savjetnik hematologa A
Podaci o autoru Autorska prava i licenca Izjava o odricanju odgovornosti
Ovaj su članak citirali drugi članci u PMC-u.
Sažetak
Trombociti imaju vrlo važnu ulogu u fiziološkoj hemostazi i stvaranju tromba. Agregacija trombocita ključni je patofiziološki faktor u razvoju arhemijskih ishemijskih događaja, uključujući bolest koronarnih arterija, cerebrovaskularne nesreće i periferne arterijske bolesti. Kao takva, antiagregacijska terapija ima vrlo važnu ulogu u sprečavanju ponavljajućih događaja kod osoba koje su pogođene jednim od ovih stanja. Donedavno je repertoar terapije protiv trombocita bio ograničen na aspirin i klopidogrel. Međutim, ovaj krajolik se dramatično promijenio s pojavom novijih i snažnijih sredstava, prasugrela i ticagrelora, kao i antagonista glikoproteina IIb / IIIa. Ovaj armamentarium vjerojatno će se proširiti s pojavom antagonista receptora-aktiviranog proteazom i intravenskog kagrelora.
KLJUČNE RIJEČI: Trombociti, antitrombocitni aspirin, klopidogrel, trombociti, prasugrel, ticagrelor
Uvod
Trombociti imaju vrlo važnu ulogu u fiziološkoj hemostazi i stvaranju tromba. Agregacija trombocita ključni je patofiziološki faktor u razvoju arterijskih ishemijskih događaja, uključujući koronarnu bolest arterija, cerebrovaskularne nesreće i periferne arterijske bolesti. Kao takva, antiagregacijska terapija ima vrlo važnu ulogu u sprečavanju ponavljajućih događaja kod osoba koje su pogođene jednim od ovih stanja. Donedavno je repertoar terapije protiv trombocita bio ograničen na aspirin i klopidogrel. Međutim, ovaj krajolik se dramatično promijenio s pojavom novijih i snažnijih sredstava, prasugrela i ticagrelora, kao i antagonista glikoproteina IIb / IIIa. Ovaj armamentarium vjerojatno će se proširiti daljnjim pojavom antagonista receptora-aktiviranih receptora-1 (PAR-1) i intravenskog kagrelora.
Za potrebe ovog pregleda, lijekovi protiv trombocita su razvrstani u tri ovisno o dolasku na tržište - prošlost, sadašnjost i budućnost.
Prošlost
Aspirin djeluje kao sredstvo protiv trombocita ireverzibilno blokira enzim ciklooksigenaza-1 (COX-1) unutar trombocita.
Ovaj enzim je potreban za stvaranje tromboksana A2, snažnog aktivatora trombocita iz arahidonske kiseline.
Aspirin također inhibira COX-2 što objašnjava dio njegovih protuupalnih svojstava, iako je također predloženo nekoliko drugih mehanizama, uključujući smanjenu proizvodnju prostaglandina i one koji uključuju imunološke stanice i citokine.
Gastrointestinalne nuspojave aspirina uzrokovane su inhibicijom COX-1. Maksimalni antitrombotski učinak aspirina postiže se dnevnom dozom od 75–100 mg, bez daljnjih koristi od povećanja doze, iako se rizik od krvarenja povećava s dozom.
Budući da aspirin selektivno cilja COX put, aktivacija trombocita može se nastaviti drugim putovima, što zahtijeva potrebu za dodatnim agensima poput klopidogrela za punu antitrombotsku funkciju.
Klopidogrel pripada klasi lijekova koji su svrstani u antagoniste adenosin-difosfata (ADP).
ADP je važan sudionik u procesu agregacije trombocita kroz interakciju s dva puringerna receptora na trombocitima koji se nazivaju P2Y 1 i P2Y 12, a posljednji imaju specifičniju distribuciju u tkivu.
Prvi inhibitor receptora P2Y 12 odobren za upotrebu bio je tiklopidin, međutim bio je povezan s nepovoljnim hematološkim učincima. Klopidogrel je druga generacija blokatora P2Y 12 koji zahtijeva svoj učinak protiv trombocita.
Potreba za aktivnim stvaranjem metabolita može uzrokovati odlaganje svojstava antiagregacijskih sloga što zahtijeva dozu punjenja da bi se postigla maksimalna inhibicija trombocita.
Klopidogrel se metabolizira u njegov aktivni metabolit dijelom jetrenim enzimom CYP2C19. Stoga bi se trebala izbjegavati uporaba istodobnih lijekova koji inhibiraju ovaj enzim (npr. Omeprazol, fluoksetin, flukonazol i karbamazepin).
Nedavno opisani genetski polimorfizmi CYP2C19 također utječu na dostupnost ili odgođen odgovor na aktivni metabolit lijekova metaboliziranih ovim enzimom.
Pogledajte tablicu Tablica 11 za farmakoloških svojstava.
Sadašnjost
Dipiridamol je uveden u 1960-ima kao koronarni vazodilatator. Budući da je pokazano da inhibira unos adenozina, snažnog agregatora trombocita, njegova antitrombocitna uloga je dodatno istražena.
To je mehanizam djelovanja inhibicije fosfodiesteraze (PDE) posredovane stanicama i uzimanjem adenozina, koji dovode do inhibicije agregacije trombocita.
Peroralni unos dipiridamola zahtijeva niski pH za apsorpciju (može izazvati probleme s istodobnom terapijom antacidima).
Priprema ovog lijeka s produljenim oslobađanjem danas je omiljena kao sredstvo protiv trombocita u prevenciji ishemijskog moždanog udara u kombinaciji s aspirinom.
Dipiridamol zbog svojih vazodilatacijskih svojstava može izazvati hipotenziju (pažljivo s antihipertenzivima) i pogoršanje koronarne bolesti srca.
Također može pogoršati miasteniju gravis interakcijom s inhibitorima kolinesteraze.
Cilostazol djeluje selektivno inhibirajući PDE tip III i prvotno je odobren za liječenje isprekidane klaudikacije.
Iako ima antiplateletnih svojstva, treba ga uvijek dati u kombinaciji s drugim sredstvom poput aspirina ili klopidogrela u bolesnika s povremenim šepanjem.
Smjernice Američkog koledža za prsni koš preporučuju dodavanje cilostazola aspirinu ili klopidogrelu pacijentima koji imaju vatrostalnu klaudikaciju unatoč terapiji vježbanja i prestanku pušenja.
U azijskim se zemljama ovaj lijek primjenjuje uz dvostruka antiagregacijska sredstva kod bolesnika koji primaju koronarne stente i kao alternativa aspirinu u bolesnika s ishemijskim moždanim udarom (manji hemoragični rizik u usporedbi s aspirinom).
Najčešće prijavljene nuspojave cilostazola su glavobolja i proljev.
To je mehanizam djelovanja inhibicije fosfodiesteraze (PDE) posredovane stanicama i uzimanjem adenozina, koji dovode do inhibicije agregacije trombocita.
Peroralni unos dipiridamola zahtijeva niski pH za apsorpciju (može izazvati probleme s istodobnom terapijom antacidima).
Priprema ovog lijeka s produljenim oslobađanjem danas je omiljena kao sredstvo protiv trombocita u prevenciji ishemijskog moždanog udara u kombinaciji s aspirinom.
Dipiridamol zbog svojih vazodilatacijskih svojstava može izazvati hipotenziju (pažljivo s antihipertenzivima) i pogoršanje koronarne bolesti srca.
Također može pogoršati miasteniju gravis interakcijom s inhibitorima kolinesteraze.
Cilostazol djeluje selektivno inhibirajući PDE tip III i prvotno je odobren za liječenje isprekidane klaudikacije.
Iako ima antiplateletnih svojstva, treba ga uvijek dati u kombinaciji s drugim sredstvom poput aspirina ili klopidogrela u bolesnika s povremenim šepanjem.
Smjernice Američkog koledža za prsni koš preporučuju dodavanje cilostazola aspirinu ili klopidogrelu pacijentima koji imaju vatrostalnu klaudikaciju unatoč terapiji vježbanja i prestanku pušenja.
U azijskim se zemljama ovaj lijek primjenjuje uz dvostruka antiagregacijska sredstva kod bolesnika koji primaju koronarne stente i kao alternativa aspirinu u bolesnika s ishemijskim moždanim udarom (manji hemoragični rizik u usporedbi s aspirinom).
Najčešće prijavljene nuspojave cilostazola su glavobolja i proljev.
Prevalencija lošeg odgovora na klopidogrel dovela je do razvoja blokatora receptora P2Y 12 , prasugrela i ticagrelora.
Prasugrel nakon oralnog unosa također treba jetreni metabolizam da bi stvorio svoj aktivni metabolit, iako je, u usporedbi s klopidogrelom, učinkovitiji, što rezultira većom bioraspoloživošću, bržim početkom djelovanja i što je najvažnije, snažnijim učinkom protiv trombocita.
Doza od 60 mg postiže inhibiciju trombocita počevši od 30 minuta, a maksimalna u vremenu od 2 do 4 sata kod bolesnika sa stabilnom koronarnom bolešću (odgađa akutni infarkt miokarda, vjerojatno uslijed sporije apsorpcije probavnog sustava).
Klinička učinkovitost prasugrela ispitivana je u TRITON-TIMI 38, s prednostima u odnosu na klopidogrel jasno vidljive u bolesnika s miokardnim infarktom elevacije ST (događaji 9,7% za klopidogrel u usporedbi s 7,4% za prasugrel) i onima s rekurentnim događajima, iako jasna prednost nije bila primijećena kod starijih osoba (≥75 godina) i osoba težih od 60 kg (savjetuje se smanjenje doze na 5 mg).
Imao je negativan utjecaj na one s poviješću moždanog udara ili prolaznim ishemijskim napadom.
Ticagrelor je drugi oralno aktivni blokator receptora P2Y 12, ali za razliku od klopidogrela i prasugrela ne zahtijeva metaboličku aktivaciju i reverzibilno inhibira receptor.
Spomenuti lijekovi nepovratni su inhibitori, što zbog trombocita je anukleat, znači da su inhibirani tijekom njihovog životnog vijeka u cirkulaciji; ali učinak lijeka opada kako nastaju novi trombociti. Primijenjen dva puta dnevno, ticagrelor, sličan prasugrelu, ima brzi početak djelovanja, jači je od klopidogrela, ali ima i prednost bržeg kompenziranja djelovanja.
Također inhibira stanični unos adenozina što može dovesti do učinaka poput osjećaja dispneje. Smatra se da inhibicija ponovne pohrane adenozina doprinosi i protuupalnim i antiagregacijskim učincima ticagrelora.
Na temelju ispitivanja faze III PLATO, ticagrelor je odobren za liječenje i sprječavanje trombotskih događaja u bolesnika s akutnim koronarnim sindromom (ACS), bez obzira da li je odabrana invazivna ili neinvazivna strategija iako nije u bolesnika sa stabilnom bolešću.
Budući da može potaknuti aritmiju, ticagrelor se ne smije primjenjivati u bolesnika s bradikardijom, sindromom bolesnog sinusa ili atrioventrikularnim blokom.
Također, pacijenti koji primaju lijekove metabolizirane enzimima CYP3A4 i CYP3A5 trebali bi izbjegavati ovo sredstvo (npr. Klaritromicin, digoksin, simvastatin).
Također je bolje izbjegavati bolesnike s astmom ili kroničnom opstruktivnom plućnom bolešću, kao i one s poviješću hiperuricemije, jer može povećati razinu mokraćne kiseline.
Razine kreatinina mogu se povećati nakon započinjanja s ticagrelorom. 16
Glikoprotein IIb / IIIa receptor je uključen u agregaciju trombocita posredovanu pretežno fibrinogenom. Trenutno su dostupna tri parenteralna inhibitora glikoproteina IIb / IIIa (GPI): abciksimab, eptifibatid i tirofiban.
Posljednja dva imaju kraći poluživot i izlučuju se putem bubrega, dok abciksimab ima veći afinitet za receptor i ne zahtijeva prilagođavanje doze kod oštećenja bubrega. Uz dostupnost moćnih antiagregacijskih sredstava u oralnoj formulaciji, primjena GPI trenutno je ograničena na bolesnike s nestabilnom anginom koji zahtijevaju perkutanu koronarnu intervenciju (PCI) i ne mogu se prethodno liječiti blokatorom P2Y12. Međutim, zbog brzog početka djelovanja i relativno brzog uklanjanja (otprilike 4 sata), GPI se sve više smatraju "premošćivajućom" strategijom za pacijente na dvostrukoj antiagregacijskoj terapiji koja treba kirurške zahvate. U okruženju peri-postupka prekid terapije antiagregata tijekom dužeg razdoblja može biti povezan s porastom tromboze.
U ovom se području provode nasumična kontrolirana ispitivanja.
Prasugrel nakon oralnog unosa također treba jetreni metabolizam da bi stvorio svoj aktivni metabolit, iako je, u usporedbi s klopidogrelom, učinkovitiji, što rezultira većom bioraspoloživošću, bržim početkom djelovanja i što je najvažnije, snažnijim učinkom protiv trombocita.
Doza od 60 mg postiže inhibiciju trombocita počevši od 30 minuta, a maksimalna u vremenu od 2 do 4 sata kod bolesnika sa stabilnom koronarnom bolešću (odgađa akutni infarkt miokarda, vjerojatno uslijed sporije apsorpcije probavnog sustava).
Klinička učinkovitost prasugrela ispitivana je u TRITON-TIMI 38, s prednostima u odnosu na klopidogrel jasno vidljive u bolesnika s miokardnim infarktom elevacije ST (događaji 9,7% za klopidogrel u usporedbi s 7,4% za prasugrel) i onima s rekurentnim događajima, iako jasna prednost nije bila primijećena kod starijih osoba (≥75 godina) i osoba težih od 60 kg (savjetuje se smanjenje doze na 5 mg).
Imao je negativan utjecaj na one s poviješću moždanog udara ili prolaznim ishemijskim napadom.
Ticagrelor je drugi oralno aktivni blokator receptora P2Y 12, ali za razliku od klopidogrela i prasugrela ne zahtijeva metaboličku aktivaciju i reverzibilno inhibira receptor.
Spomenuti lijekovi nepovratni su inhibitori, što zbog trombocita je anukleat, znači da su inhibirani tijekom njihovog životnog vijeka u cirkulaciji; ali učinak lijeka opada kako nastaju novi trombociti. Primijenjen dva puta dnevno, ticagrelor, sličan prasugrelu, ima brzi početak djelovanja, jači je od klopidogrela, ali ima i prednost bržeg kompenziranja djelovanja.
Također inhibira stanični unos adenozina što može dovesti do učinaka poput osjećaja dispneje. Smatra se da inhibicija ponovne pohrane adenozina doprinosi i protuupalnim i antiagregacijskim učincima ticagrelora.
Na temelju ispitivanja faze III PLATO, ticagrelor je odobren za liječenje i sprječavanje trombotskih događaja u bolesnika s akutnim koronarnim sindromom (ACS), bez obzira da li je odabrana invazivna ili neinvazivna strategija iako nije u bolesnika sa stabilnom bolešću.
Budući da može potaknuti aritmiju, ticagrelor se ne smije primjenjivati u bolesnika s bradikardijom, sindromom bolesnog sinusa ili atrioventrikularnim blokom.
Također, pacijenti koji primaju lijekove metabolizirane enzimima CYP3A4 i CYP3A5 trebali bi izbjegavati ovo sredstvo (npr. Klaritromicin, digoksin, simvastatin).
Također je bolje izbjegavati bolesnike s astmom ili kroničnom opstruktivnom plućnom bolešću, kao i one s poviješću hiperuricemije, jer može povećati razinu mokraćne kiseline.
Razine kreatinina mogu se povećati nakon započinjanja s ticagrelorom. 16
Glikoprotein IIb / IIIa receptor je uključen u agregaciju trombocita posredovanu pretežno fibrinogenom. Trenutno su dostupna tri parenteralna inhibitora glikoproteina IIb / IIIa (GPI): abciksimab, eptifibatid i tirofiban.
Posljednja dva imaju kraći poluživot i izlučuju se putem bubrega, dok abciksimab ima veći afinitet za receptor i ne zahtijeva prilagođavanje doze kod oštećenja bubrega. Uz dostupnost moćnih antiagregacijskih sredstava u oralnoj formulaciji, primjena GPI trenutno je ograničena na bolesnike s nestabilnom anginom koji zahtijevaju perkutanu koronarnu intervenciju (PCI) i ne mogu se prethodno liječiti blokatorom P2Y12. Međutim, zbog brzog početka djelovanja i relativno brzog uklanjanja (otprilike 4 sata), GPI se sve više smatraju "premošćivajućom" strategijom za pacijente na dvostrukoj antiagregacijskoj terapiji koja treba kirurške zahvate. U okruženju peri-postupka prekid terapije antiagregata tijekom dužeg razdoblja može biti povezan s porastom tromboze.
U ovom se području provode nasumična kontrolirana ispitivanja.
Budućnost
Sva tri blokatora receptora P2Y 12 koji se primjenjuju oralno imaju nedostatak što ih se ne može davati nesvjesnim ili intubiranim pacijentima ili onima s hemodinamičkim kompromisom, kao i ako pacijent opetovano povraća. Cangrelor je intravenska formulacija koja se reverzibilno veže za P2Y 12 receptor i postiže učinkovite koncentracije u roku od nekoliko minuta.
Također ima prednost stabilne farmakokinetike, vrlo kratkog poluživota (5 minuta) - što znači brzo odstupanje od djelovanja i razine agregacije trombocita prije liječenja postignute u roku od 30–60 minuta.
Moguća upotreba kangrelora bit će premošćavajuća strategija kod pacijenata s ACS-om kojima će možda trebati hitna operacija zaobilaznih presada koronarnih arterija.
Trombin na mjestima vaskularne ozljede regrutira trombocite u tvorbu hemostatskog čepa. Taj proces posreduju receptori poznati kao receptori aktivirani proteazom (PARS). Vorapaxar je antagonist PAR-a i odobren je za primjenu u smanjenju trombotskih događaja kod pacijenata s anamnezom infarkta miokarda ili periferne arterijske bolesti, u kombinaciji sa standardnom antiplatničnom terapijom.
Njegova je primjena kontraindicirana u bolesnika s moždanim udarom, prolaznim ishemijskim napadom ili intrakranijalnim krvarenjem zbog povećanog rizika od velikih krvarenja.
Također ima prednost stabilne farmakokinetike, vrlo kratkog poluživota (5 minuta) - što znači brzo odstupanje od djelovanja i razine agregacije trombocita prije liječenja postignute u roku od 30–60 minuta.
Moguća upotreba kangrelora bit će premošćavajuća strategija kod pacijenata s ACS-om kojima će možda trebati hitna operacija zaobilaznih presada koronarnih arterija.
Trombin na mjestima vaskularne ozljede regrutira trombocite u tvorbu hemostatskog čepa. Taj proces posreduju receptori poznati kao receptori aktivirani proteazom (PARS). Vorapaxar je antagonist PAR-a i odobren je za primjenu u smanjenju trombotskih događaja kod pacijenata s anamnezom infarkta miokarda ili periferne arterijske bolesti, u kombinaciji sa standardnom antiplatničnom terapijom.
Njegova je primjena kontraindicirana u bolesnika s moždanim udarom, prolaznim ishemijskim napadom ili intrakranijalnim krvarenjem zbog povećanog rizika od velikih krvarenja.
Indikacije za terapiju protiv trombocita
Indikacija za lijekove koji djeluju protiv trombocita i preporučuje Nacionalni institut za izvrsnost u zdravstvu i njezi i smjernice American Heart Association navedene su u nastavku.
Akutni koronarni sindrom
Indikacija za lijekove koji djeluju protiv trombocita i preporučuje Nacionalni institut za izvrsnost u zdravstvu i njezi i smjernice American Heart Association navedene su u nastavku.
Akutni koronarni sindrom
- Svi bolesnici trebali bi se dugoročno liječiti aspirinom u malim dozama (75 mg dnevno). U slučaju netolerancije na aspirin, klopidogrel (75 mg dnevno) je alternativa.
- Za pacijente s točno određenom ili vjerovatno ne-povišenom ACS-om s porastom ST kod kojih je odabrana početna invazivna ili ishemijom vođena strategija: aspirin s inhibitorom P2Y 12 ; klopidogrel 300 ili 600 mg ili ticagrelor 180 mg.
- Za bolesnike s ACS-om s povišenjem ST koji su podvrgnuti PCI sa stentiranjem: aspirin s inhibitorom P2Y 12 , klopidogrel 300 ili 600 mg ili ticagrelor 180 mg ili prasugrel 60 mg.
- Održavanje aspirina 75 mg / dan nastavlja se neograničeno, plus inhibitora P2Y 12 do 12 mjeseci: klopidogrela 75 mg / dan ili ticagrelora 90 mg dva puta dnevno (ili PCI sa stentom prasugrela 10 mg / dan).
Antiplatelet and Antithrombotic Activities
Mauritia flexuosa (Aguaje)
Presents
In Vitro and In Vivo
Abstract
Fruit from the palm Mauritia flexuosa is one of the most important species in Peru, Venezuela, Brazil, Colombia, Bolivia, and Guyana. The present study aimed to investigate the antiplatelet and antithrombotic activities of oil extracted from Mauritia flexuosa. The fatty acid contents were determined by gas chromatography—mass spectrometry. Oil extract of peel of Mauritia flexuosa was extracted by soxhlet extraction. The oil extract inhibited platelet secretion and aggregation induced by ADP, collagen, and TRAP-6 by a concentration-dependent way (0.1 to 1 mg/mL) without the participation of the adenylyl cyclase pathway and diminished platelet rolling and firm adhesion under flow conditions. Furthermore, the oil extract induced a marked increase in the rolling speed of leukocytes retained on the platelet surface, reflecting a reduction of rolling and less adhesion. At the concentrations used, the oil extract significantly decreased platelet release of sP-selectin, an atherosclerotic-related inflammatory mediator. Oil extract inhibited thrombus growth at the same concentration as that of aspirin, a classical reference drug. Finally, the data presented herein also demonstrate for the first time to our knowledge the protective effect of oil extracted from Mauritia flexuosa on platelet activation and thrombosis formation.
1. Introduction
Platelets play an essential role in the pathogenesis of cardiovascular diseases (CVD). In this way, the antiplatelet therapy has been used for a long time in an effort to prevent CVD
Diets rich in fruits and vegetables (F&V), part of the so-called Mediterranean diet, reduce blood pressure and the risk of adverse cardiovascular events . In addition, the regular consumption of fruits and vegetables has been shown to be beneficial in terms of reducing the platelet activity. Thus, in primary prevention trials, an energy-unrestricted Mediterranean diet supplemented with extra-virgin olive oil or nuts resulted in a substantial reduction in the risk of major cardiovascular events among high-risk persons. Even, a higher consumption of vegetable oils rich in monounsaturated and polyunsaturated fatty acids (sunflower, corn, canola, and olive oils) has been associated with lower concentrations of inflammatory mediators, oxidative damage, and an increase of the healthy cholesterol (HDL cholesterol).
Of the estimated 30 million species of plants found in the Amazon, only a few have been studied and identified so far, based on popular knowledge and scientific studies. As the use of Amazonian vegetable oils has increased in recent years, it is important to acquire a better knowledge of their properties in order to optimize the use of these materials . The burití, moriche, canangucha, mirití, or aguaje (Mauritia flexuosa) is a species of palm belonging to the Arecaceae family. It is one of the most important of tropical America, found in Peru, Venezuela, Brazil, Colombia, Bolivia, and Guyana. Fruit from the palm Mauritia flexuosa is harvested for subsistence and commercial purposes . Oil extracted from fruits of Mauritia flexuosa is used in cooking and is rich in monounsaturated fatty acids and natural antioxidants . Also, Mauritia flexuosa oil has found applications in the cosmetic industry due to its emollient properties and can be used as an adjuvant in sun protection. Yet, the protective effect of Mauritia flexuosa on platelet activation and thrombosis formation remains to be fully elucidated. The present study aimed to investigate the antiplatelet and antithrombotic activities of oil extract from peel of fruits of Mauritia flexuosa.
2. materials and methods
2.1. Reagents
Sodium chloride (p.a.) was obtained from Arquimed (Santiago, Chile). Adenosine 5′-diphosphate (ADP), thrombin receptor activator peptide 6 (TRAP-6), calcein-AM, collagen, acetylsalicylic acid (ASA), bovine serum albumin (BSA), SQ22536 (an adenylyl cyclase inhibitor), rose bengal, prostaglandin E1 (PGE1), rhodamine 6G, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Luciferase-luciferin reagent was obtained from Chrono-Log corp (Havertown, PA) and microfluidic chambers were from Bioflux (Fluxion, San Francisco, CA, USA).
2.2. Vegetable Oil
For the Soxhlet extraction we used a peel from fruits of Mauritia flexuosa purchased from local markets in Colombia. The peel was separated from the pulp in a kitchen food processor, washed with water, and frozen until used in order to avoid any modification due to exposure to heat or light. Then, the peel was subjected to Soxhlet extraction using hexane as a solvent for 8 hours, removing the solvent by distillation under reduced pressure at 37°C. After the evaporation of the solvent, yellow, limpid oil was obtained (yield 13%). The oil extract from Mauritia flexuosa was frozen until it was used. For in vitro and in vivo studies the oil sample was mixed homogenously in DMSO 0.3%.
2.3. Fatty Acid Determination by Gas Chromatography
The fatty acid profile was determined as fatty acid methyl esters by gas chromatography-mass spectrometry. The methyl esters were prepared using the method described by Morrison and Smith [15]. Separation of fatty acid esters was performed using an Agilent 7890A Gas Chromatograph equipped with an Agilent J&W DB-5ms capillary column (30 m × 0.32 mm × 0.5 μm). The column temperature was programmed at 100°C for 1 min, then it was increased to 200°C at 5°C/min, 250°C at 3°C/min, and 300°C at 5°C/min. Helium was used as carrier gas with constant linear velocity of 14 mL/min. The injector temperature was set at 150°C, in splitless mode. The temperature of the mass spectrometer detector quadrupole was 150°C and for the source 230°C, in an acquisition mode of scan.
2.4. Preparation of Human Platelet Suspensions
Venous blood samples were taken from two young healthy volunteers—who previously signed informed consents—in 3.2% citrate tubes (9 : 1 v/v) by phlebotomy with vacuum tube system (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). The protocol was authorized by the ethic committee of Universidad de Talca in accordance with the Declaration of Helsinki (approved by the 18th World Medical Assembly in Helsinki, Finland, 1964). Tubes were centrifuged (DCS-16 Centrifugal Presvac RV) at 240 g for 10 minutes to obtain platelet-rich plasma (PRP). PRP was adjusted to 200 × 109 platelets/L with platelet-poor plasma (PPP) obtained by centrifugation of the original tubes at 650 g (10 minutes). Washed platelets were prepared in Tyrode-HEPES buffer containing 50 ng/mL PGE1 and 1 mmol/L ACD-A, pH 7.4, at a concentration of 200 × 109 platelets/L. Platelet counts were performed in a hematologic counter (Bayer Advia 60 Hematology System, Tarrytown, NY, USA).
2.5. Assessment of Platelet Activation by Quantization of Soluble P-Selectin
Washed platelets were pretreated with DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 and 1 mg/mL) for 15 min at 37°C and then stimulated by thrombin (2 U/mL) for 45 min at 37°C. Supernatants were collected after centrifugation (2.000 g, 10 min, 4°C) and stored at −70°C until sP-selectin analysis (Invitrogen Corporation, CA, USA).
2.6. Measurement of Platelet SecretionSecretion was analyzed after preincubation of platelets (480 μL of PRP adjusted to 200 × 109 platelets/L) with 20 µL of DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 to 1 mg/mL) for 3 min prior to the addition of luciferin/luciferase (50 μL) and agonist (20 μL) (ADP 8 µmol/L, collagen 1.5 μg/mL, or TRAP-6 30 µmol/L). Platelet secretion was measured at real time over a 6 min period in a lumi-aggregometer (Chrono-Log, Havertown, PA, USA) at 37°C with stirring (150 g). Controls were run with DMSO 0.3% and the average control secretion was taken as 100%.
2.7. Platelet Aggregation Assay
Platelet aggregation was monitored by light transmission according to Born and Cross [16], using a lumi-aggregometer. Briefly, 480 μL of PRP (200 × 109 platelets/L) was preincubated with 20 μL of DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 to 1 mg/mL) for 3 min. Thereafter, 20 μL of agonist (ADP 8 µmol/L, collagen 1.5 μg/mL, or TRAP-6 30 µmol/L) was added and platelet aggregation was registered during 6 min. All measurements were performed in triplicate. The results of platelet aggregation (maximal amplitude (%)) were determined by the software AGGRO/LINK (Chrono-Log, Havertown, PA, USA). Controls were run with DMSO 0.3% and the average control aggregation was taken as 100%.
2.8. Effect of SQ22536 on ADP-Induced Platelet Aggregation
To elucidate whether the oil extract antiaggregant activity was mediated by the adenylyl cyclase pathway, PRP was pretreated with SQ22536 (200 and 400 µmol/L) for 3 min before the addition of oil extract (1 mg/mL). Then, platelet aggregation was challenged by ADP 8 µmol/L. Platelets firstly exposed to SQ22536 and then ADP were used for control purposes.
2.9. Analysis of Platelet Rolling and Firm Adhesion under Controlled Flow Conditions
Experiments under flow were performed in a BioFlux-200 flow system (Fluxion, San Francisco, CA, USA) with high shear plates (48 wells, 0–20 dyne/cm2) [17]. The microfluidic chambers were coated with 20 μL of collagen 200 µg/mL at a wall shear rate of 200 s−1 for 1 hour. The plaque coating was allowed to dry at room temperature (RT) for one hour. Thereafter, channels were perfused with phosphate buffered saline (PBS) for 10 min to remove the interface and blocked with BSA 5% for 10 min (RT, wall shear rate 200 s−1). Whole blood, anticoagulated with sodium citrate, was labeled with calcein-AM (4 μmol/L) and incubated with DMSO 0.3%, PGE1 (0.02 mmol/L), or oil extract (1 mg/mL) for 15 min (RT) before being added into the inlet of the well. Chambers were perfused for 10 min at room temperature at a wall shear rate of 1000 s−1. The plates were mounted on the stage of an inverted fluorescence microscope (TE200, NIKON, Japan).
Platelet deposition was observed and recorded at real time with a CCD camera (QICAM, QIMaging, Surrey, BC, Canada). Real-time visualization between platelets and collagen interactions was performed using a bright field and fluorescence microscopy. Platelets and collagen interaction over a 30 s interval was defined as rolling whereas platelet immobilization for more than 30 s was defined as firm adhesion. For each flow experiment, fluorescent images were analyzed off-stage by quantifying the area covered by platelets with the ImageJ software (version 1.26t, NIH, USA).
2.10. Leukocyte Rolling over Collagen-Bound Platelet Monolayer under Controlled Flow
The collagen-coated microfluidic chambers were rinsed in PBS buffer and perfused with whole blood (anticoagulated with sodium citrate), labeled with calcein-AM 4 μmol/L at a shear rate of 200 s−1 for 10 min at RT. Under these conditions, a homogeneous carpet of spread platelets was formed. The remaining blood was washed out with DMSO 0.3%, PGE1 (0.02 mmol/L), or oil extract (1 mg/mL) for 3 min. Thereafter, leukocytes rolling and firm adhesion were visualized on the platelet layer with an inverted fluorescence microscope. To this end, leukocytes were previously labeled with rhodamine 6G (50 μL of 0.05%). Digital movies were captured, and translocation velocity was calculated by image analysis (ImageJ)
2.11. In Vivo Murine Model of Thrombosis
Mice (12–16 weeks old) were anesthetized with a combination of tribromoethanol (270 mg/kg) and xylazine (13 mg/kg). The mesentery was exposed by performing a central incision in the abdomen, permitting the visualization of thrombus development in mesenteric vessels. Thrombosis was induced by injection of 50 mg/kg rose bengal through the tail vein followed by illumination of the exposed mesenteric area with a 1.5 mW green light laser (532 nm). Blood flow was monitored for 60 min and stable occlusion was defined as a blood flow of 0 mL/min for 3 min. DMSO 0.3% (control group, n = 3), ASA (200 mg/Kg, n = 3), or oil extract (200 mg/kg, n = 3), was administered intraperitoneally 30 min before the experiment. Rectal temperatures were similar and within the physiological range among all experimental animals throughout the experimental period.
2.12. Statistical Analysis
Data were analyzed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA. and expressed as mean ± standard error of mean (SEM). Three or more independent experiments were performed for the different assays. Fifty-percent inhibitory concentration (IC50) of oil extract against agonist-induced platelet function was calculated by a regression analysis. Differences between groups were analyzed by Student's paired or unpaired t-test and one-way analysis of variance (ANOVA) using Tukey's post hoc test. P values <0.05 were considered significant.
2.1. Reagents
Sodium chloride (p.a.) was obtained from Arquimed (Santiago, Chile). Adenosine 5′-diphosphate (ADP), thrombin receptor activator peptide 6 (TRAP-6), calcein-AM, collagen, acetylsalicylic acid (ASA), bovine serum albumin (BSA), SQ22536 (an adenylyl cyclase inhibitor), rose bengal, prostaglandin E1 (PGE1), rhodamine 6G, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Luciferase-luciferin reagent was obtained from Chrono-Log corp (Havertown, PA) and microfluidic chambers were from Bioflux (Fluxion, San Francisco, CA, USA).
2.2. Vegetable Oil
For the Soxhlet extraction we used a peel from fruits of Mauritia flexuosa purchased from local markets in Colombia. The peel was separated from the pulp in a kitchen food processor, washed with water, and frozen until used in order to avoid any modification due to exposure to heat or light. Then, the peel was subjected to Soxhlet extraction using hexane as a solvent for 8 hours, removing the solvent by distillation under reduced pressure at 37°C. After the evaporation of the solvent, yellow, limpid oil was obtained (yield 13%). The oil extract from Mauritia flexuosa was frozen until it was used. For in vitro and in vivo studies the oil sample was mixed homogenously in DMSO 0.3%.
2.3. Fatty Acid Determination by Gas Chromatography
The fatty acid profile was determined as fatty acid methyl esters by gas chromatography-mass spectrometry. The methyl esters were prepared using the method described by Morrison and Smith [15]. Separation of fatty acid esters was performed using an Agilent 7890A Gas Chromatograph equipped with an Agilent J&W DB-5ms capillary column (30 m × 0.32 mm × 0.5 μm). The column temperature was programmed at 100°C for 1 min, then it was increased to 200°C at 5°C/min, 250°C at 3°C/min, and 300°C at 5°C/min. Helium was used as carrier gas with constant linear velocity of 14 mL/min. The injector temperature was set at 150°C, in splitless mode. The temperature of the mass spectrometer detector quadrupole was 150°C and for the source 230°C, in an acquisition mode of scan.
2.4. Preparation of Human Platelet Suspensions
Venous blood samples were taken from two young healthy volunteers—who previously signed informed consents—in 3.2% citrate tubes (9 : 1 v/v) by phlebotomy with vacuum tube system (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). The protocol was authorized by the ethic committee of Universidad de Talca in accordance with the Declaration of Helsinki (approved by the 18th World Medical Assembly in Helsinki, Finland, 1964). Tubes were centrifuged (DCS-16 Centrifugal Presvac RV) at 240 g for 10 minutes to obtain platelet-rich plasma (PRP). PRP was adjusted to 200 × 109 platelets/L with platelet-poor plasma (PPP) obtained by centrifugation of the original tubes at 650 g (10 minutes). Washed platelets were prepared in Tyrode-HEPES buffer containing 50 ng/mL PGE1 and 1 mmol/L ACD-A, pH 7.4, at a concentration of 200 × 109 platelets/L. Platelet counts were performed in a hematologic counter (Bayer Advia 60 Hematology System, Tarrytown, NY, USA).
2.5. Assessment of Platelet Activation by Quantization of Soluble P-Selectin
Washed platelets were pretreated with DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 and 1 mg/mL) for 15 min at 37°C and then stimulated by thrombin (2 U/mL) for 45 min at 37°C. Supernatants were collected after centrifugation (2.000 g, 10 min, 4°C) and stored at −70°C until sP-selectin analysis (Invitrogen Corporation, CA, USA).
2.6. Measurement of Platelet SecretionSecretion was analyzed after preincubation of platelets (480 μL of PRP adjusted to 200 × 109 platelets/L) with 20 µL of DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 to 1 mg/mL) for 3 min prior to the addition of luciferin/luciferase (50 μL) and agonist (20 μL) (ADP 8 µmol/L, collagen 1.5 μg/mL, or TRAP-6 30 µmol/L). Platelet secretion was measured at real time over a 6 min period in a lumi-aggregometer (Chrono-Log, Havertown, PA, USA) at 37°C with stirring (150 g). Controls were run with DMSO 0.3% and the average control secretion was taken as 100%.
2.7. Platelet Aggregation Assay
Platelet aggregation was monitored by light transmission according to Born and Cross [16], using a lumi-aggregometer. Briefly, 480 μL of PRP (200 × 109 platelets/L) was preincubated with 20 μL of DMSO 0.3%, ASA (0.3 mmol/L), or oil extract (0.1 to 1 mg/mL) for 3 min. Thereafter, 20 μL of agonist (ADP 8 µmol/L, collagen 1.5 μg/mL, or TRAP-6 30 µmol/L) was added and platelet aggregation was registered during 6 min. All measurements were performed in triplicate. The results of platelet aggregation (maximal amplitude (%)) were determined by the software AGGRO/LINK (Chrono-Log, Havertown, PA, USA). Controls were run with DMSO 0.3% and the average control aggregation was taken as 100%.
2.8. Effect of SQ22536 on ADP-Induced Platelet Aggregation
To elucidate whether the oil extract antiaggregant activity was mediated by the adenylyl cyclase pathway, PRP was pretreated with SQ22536 (200 and 400 µmol/L) for 3 min before the addition of oil extract (1 mg/mL). Then, platelet aggregation was challenged by ADP 8 µmol/L. Platelets firstly exposed to SQ22536 and then ADP were used for control purposes.
2.9. Analysis of Platelet Rolling and Firm Adhesion under Controlled Flow Conditions
Experiments under flow were performed in a BioFlux-200 flow system (Fluxion, San Francisco, CA, USA) with high shear plates (48 wells, 0–20 dyne/cm2) [17]. The microfluidic chambers were coated with 20 μL of collagen 200 µg/mL at a wall shear rate of 200 s−1 for 1 hour. The plaque coating was allowed to dry at room temperature (RT) for one hour. Thereafter, channels were perfused with phosphate buffered saline (PBS) for 10 min to remove the interface and blocked with BSA 5% for 10 min (RT, wall shear rate 200 s−1). Whole blood, anticoagulated with sodium citrate, was labeled with calcein-AM (4 μmol/L) and incubated with DMSO 0.3%, PGE1 (0.02 mmol/L), or oil extract (1 mg/mL) for 15 min (RT) before being added into the inlet of the well. Chambers were perfused for 10 min at room temperature at a wall shear rate of 1000 s−1. The plates were mounted on the stage of an inverted fluorescence microscope (TE200, NIKON, Japan).
Platelet deposition was observed and recorded at real time with a CCD camera (QICAM, QIMaging, Surrey, BC, Canada). Real-time visualization between platelets and collagen interactions was performed using a bright field and fluorescence microscopy. Platelets and collagen interaction over a 30 s interval was defined as rolling whereas platelet immobilization for more than 30 s was defined as firm adhesion. For each flow experiment, fluorescent images were analyzed off-stage by quantifying the area covered by platelets with the ImageJ software (version 1.26t, NIH, USA).
2.10. Leukocyte Rolling over Collagen-Bound Platelet Monolayer under Controlled Flow
The collagen-coated microfluidic chambers were rinsed in PBS buffer and perfused with whole blood (anticoagulated with sodium citrate), labeled with calcein-AM 4 μmol/L at a shear rate of 200 s−1 for 10 min at RT. Under these conditions, a homogeneous carpet of spread platelets was formed. The remaining blood was washed out with DMSO 0.3%, PGE1 (0.02 mmol/L), or oil extract (1 mg/mL) for 3 min. Thereafter, leukocytes rolling and firm adhesion were visualized on the platelet layer with an inverted fluorescence microscope. To this end, leukocytes were previously labeled with rhodamine 6G (50 μL of 0.05%). Digital movies were captured, and translocation velocity was calculated by image analysis (ImageJ)
2.11. In Vivo Murine Model of Thrombosis
Mice (12–16 weeks old) were anesthetized with a combination of tribromoethanol (270 mg/kg) and xylazine (13 mg/kg). The mesentery was exposed by performing a central incision in the abdomen, permitting the visualization of thrombus development in mesenteric vessels. Thrombosis was induced by injection of 50 mg/kg rose bengal through the tail vein followed by illumination of the exposed mesenteric area with a 1.5 mW green light laser (532 nm). Blood flow was monitored for 60 min and stable occlusion was defined as a blood flow of 0 mL/min for 3 min. DMSO 0.3% (control group, n = 3), ASA (200 mg/Kg, n = 3), or oil extract (200 mg/kg, n = 3), was administered intraperitoneally 30 min before the experiment. Rectal temperatures were similar and within the physiological range among all experimental animals throughout the experimental period.
2.12. Statistical Analysis
Data were analyzed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA. and expressed as mean ± standard error of mean (SEM). Three or more independent experiments were performed for the different assays. Fifty-percent inhibitory concentration (IC50) of oil extract against agonist-induced platelet function was calculated by a regression analysis. Differences between groups were analyzed by Student's paired or unpaired t-test and one-way analysis of variance (ANOVA) using Tukey's post hoc test. P values <0.05 were considered significant.
3. results
3.1. Fatty Acid Determination by Gas Chromatography
The values obtained by gas chromatography for the chemical composition of fatty acids in the oil extract are presented in Table 1. It was observed that the oil extracted from peels of Mauritia flexuosa fruits had a high content of saturated fatty acids (59% of C8–C22), unsaturated fatty acids (37.9% of C16–C18), and a volatile fraction, which corresponds to limonene (2.7%). Within the composition of saturated fatty acids highlights the presence of isopalmitic (32%) and Stearic (19.8%) acids, while unsaturated fatty acids include oleic (33.4%) and linoleic (3.7%) acids.
The values obtained by gas chromatography for the chemical composition of fatty acids in the oil extract are presented in Table 1. It was observed that the oil extracted from peels of Mauritia flexuosa fruits had a high content of saturated fatty acids (59% of C8–C22), unsaturated fatty acids (37.9% of C16–C18), and a volatile fraction, which corresponds to limonene (2.7%). Within the composition of saturated fatty acids highlights the presence of isopalmitic (32%) and Stearic (19.8%) acids, while unsaturated fatty acids include oleic (33.4%) and linoleic (3.7%) acids.
Table 1Fatty acid compositions of oil extract from peel of Mauritia flexuosa.
Fatty acid composition (%)Peel8:0 (caprylic acid)1.0
10:0 (capric acid)0.3
12:0 (lauric acid)0.7
13:0 (tridecylic acid)0.2
14:0 (myristic acid)1.5
15:0 (valeric acid)0.5
16:0 (isopalmitic acid)32.0
16:0 (palmitic acid)1.0
14:0 (margaric acid)0.8
18:0 (stearic acid)19.8
20:0 (arachidic acid)0.8
22:0 (behenic acid)0.4
16:1 (palmitoleic acid)0.8
18:1 (oleic acid)33.4
18:2 (linoleic acid)3.7
Fatty acid composition (%)Peel8:0 (caprylic acid)1.0
10:0 (capric acid)0.3
12:0 (lauric acid)0.7
13:0 (tridecylic acid)0.2
14:0 (myristic acid)1.5
15:0 (valeric acid)0.5
16:0 (isopalmitic acid)32.0
16:0 (palmitic acid)1.0
14:0 (margaric acid)0.8
18:0 (stearic acid)19.8
20:0 (arachidic acid)0.8
22:0 (behenic acid)0.4
16:1 (palmitoleic acid)0.8
18:1 (oleic acid)33.4
18:2 (linoleic acid)3.7
In addition to the identification and relative quantification of fatty acid methyl esters the presence of monoterpene limonene was detected. Its compound has a time retention and relative abundance of 4.755 min and 2.7%, respectively. Despite the drastic conditions for the formation of methyl esters such as high temperatures and basic conditions, limonene was not degraded due to its high boiling point (175°C), also is responsible for flavour of the oil extracted from peels of Mauritia flexuosa fruits.
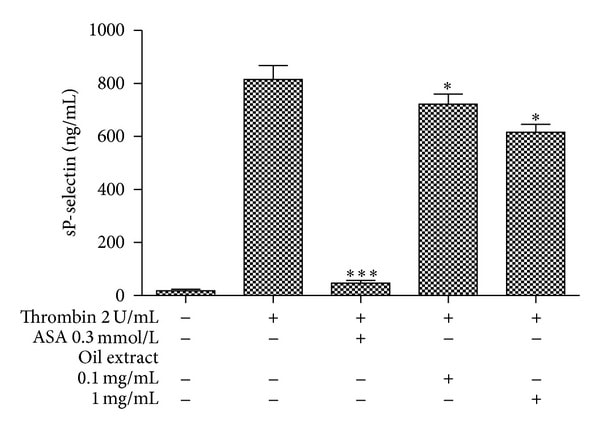
3.2. Effect of Oil Extract on sP-Selectin Platelet Release
Pretreatment of washed platelet with increasing concentrations of oil extract (0.1 and 1 mg/mL) significantly inhibited thrombin-induced sP-selectin expression up to 0.1 mg/mL. Concretely, thrombin-induced sP-selectin release was inhibited by 18 ± 2 and 29 ± 3% (P < 0.05), in the presence of oil extract at 0.1 and 1 mg/mL, respectively.
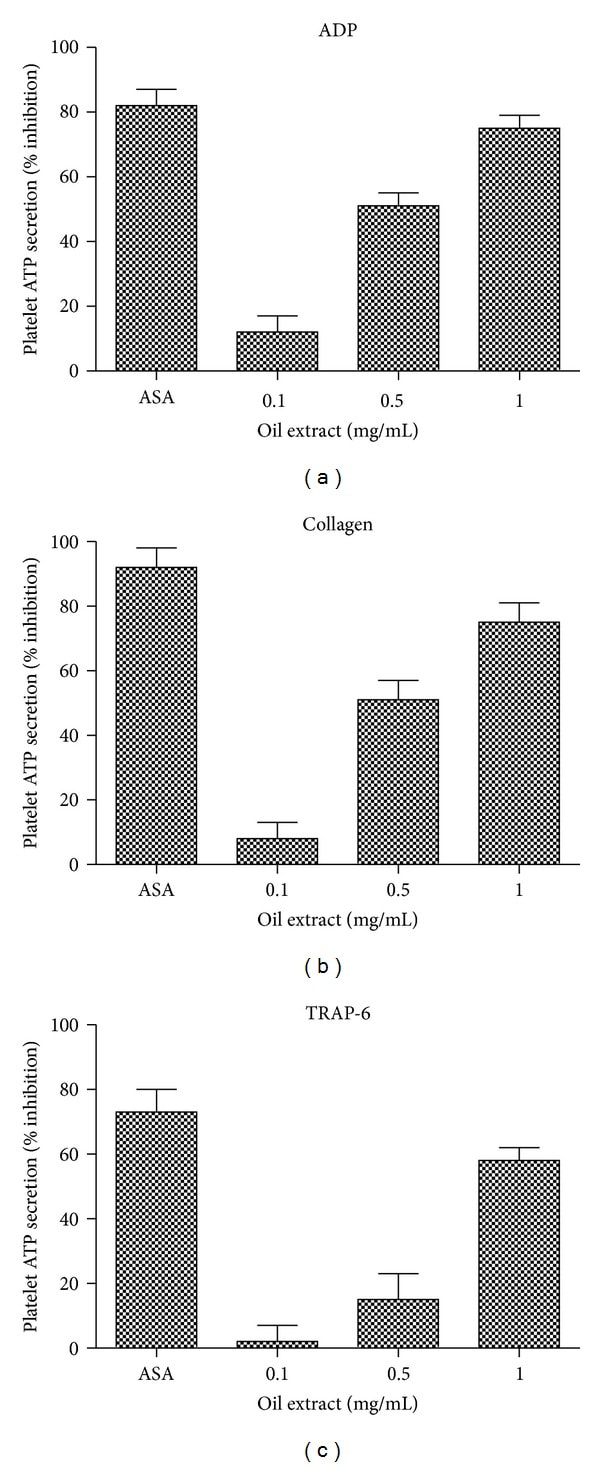
3.3. Effects of Oil Extract on Platelet ATP Secretion
The effects of oil extract on platelet ATP secretion induced by ADP, collagen, and TRAP-6 are shown in Figure 2. Oil extract showed inhibitory effects on ADP-, collagen-, and TRAP-6-induced platelet ATP secretion (P < 0.05). Oil extract inhibited ADP-induced ATP secretion, with a calculated IC50 concentration of 0.59 mg/mL. Similarly, the IC50 concentration for oil extract on collagen-induced platelet ATP-secretion was about 0.69 mg/mL, while the IC50 concentration for oil extract on TRAP-6-induced platelet ATP-secretion was about 0.93 mg/mL.
The effects of oil extract on platelet ATP secretion induced by ADP, collagen, and TRAP-6 are shown in Figure 2. Oil extract showed inhibitory effects on ADP-, collagen-, and TRAP-6-induced platelet ATP secretion (P < 0.05). Oil extract inhibited ADP-induced ATP secretion, with a calculated IC50 concentration of 0.59 mg/mL. Similarly, the IC50 concentration for oil extract on collagen-induced platelet ATP-secretion was about 0.69 mg/mL, while the IC50 concentration for oil extract on TRAP-6-induced platelet ATP-secretion was about 0.93 mg/mL.
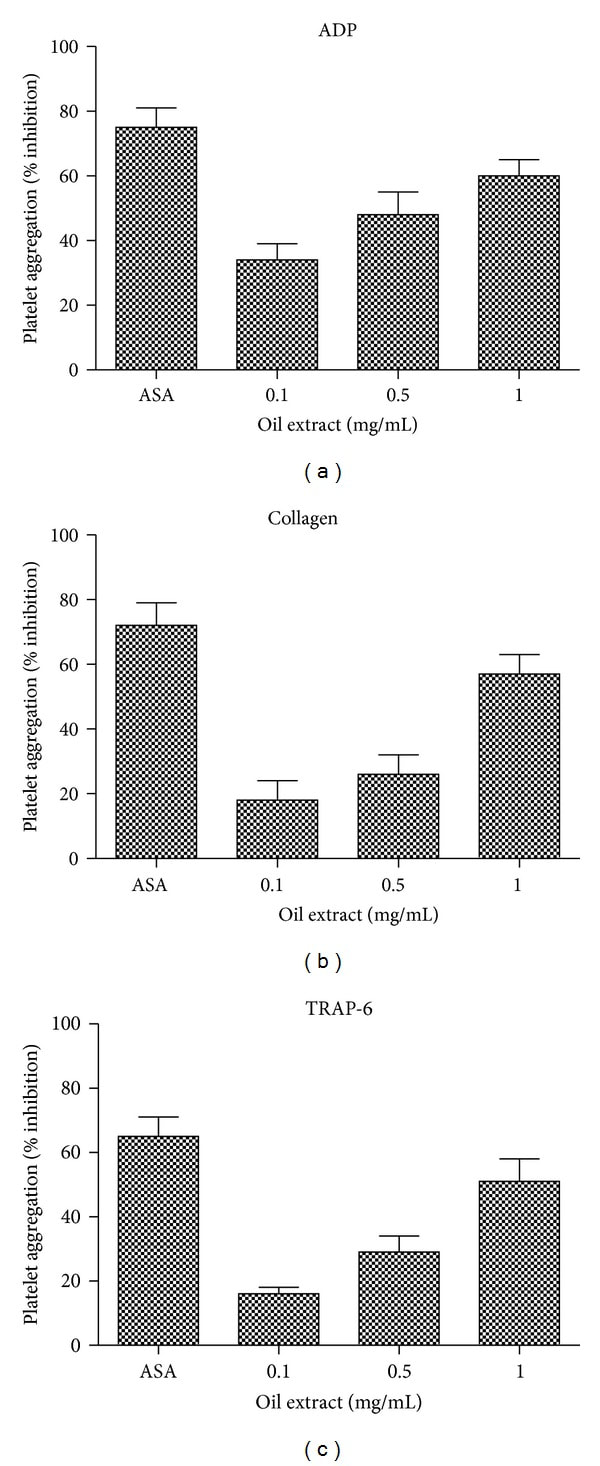
3.4. Effects of Oil Extract on Platelet Aggregation
The effects of oil extract on ADP-, collagen-, and TRAP-6-induced platelet aggregation are presented in Figure 3. Oil extract showed inhibitory effects on ADP-, collagen-, and TRAP-6-induced platelet aggregation (P < 0.05). Oil extract effectively reduced ADP-induced platelet aggregation with a 50% inhibitory concentration (IC50) of 0.65 mg/mL. Similarly, oil extract also suppressed collagen- and TRAP-6-induced platelet aggregation with IC50 of 0.93 and 0.99 mg/mL.
The effects of oil extract on ADP-, collagen-, and TRAP-6-induced platelet aggregation are presented in Figure 3. Oil extract showed inhibitory effects on ADP-, collagen-, and TRAP-6-induced platelet aggregation (P < 0.05). Oil extract effectively reduced ADP-induced platelet aggregation with a 50% inhibitory concentration (IC50) of 0.65 mg/mL. Similarly, oil extract also suppressed collagen- and TRAP-6-induced platelet aggregation with IC50 of 0.93 and 0.99 mg/mL.
3.5. SQ22536 Did Not Attenuate Effect of Oil Extract on Platelet Aggregation
To study if the inhibition of platelet function by oil extract is thought to be mediated by the stimulation of adenylate cyclase with increased intraplatelet cAMP concentrations, we incubated platelet with SQ22536 200 and 400 µmol/L (an adenylate cyclase inhibitor) and then with oil extract for platelet aggregation induced by ADP. We found that SQ22536 could not reverse the inhibitory effect of oil extract on platelet aggregation induced by ADP (P > NS). As control, SQ22536 400 µmol/L alone did not exert any effect on ADP (8 µmol/L)-induced platelet aggregation.
3.6. Oil Extract Reduces Platelet Spreading on Immobilized Collagen under Flow Conditions
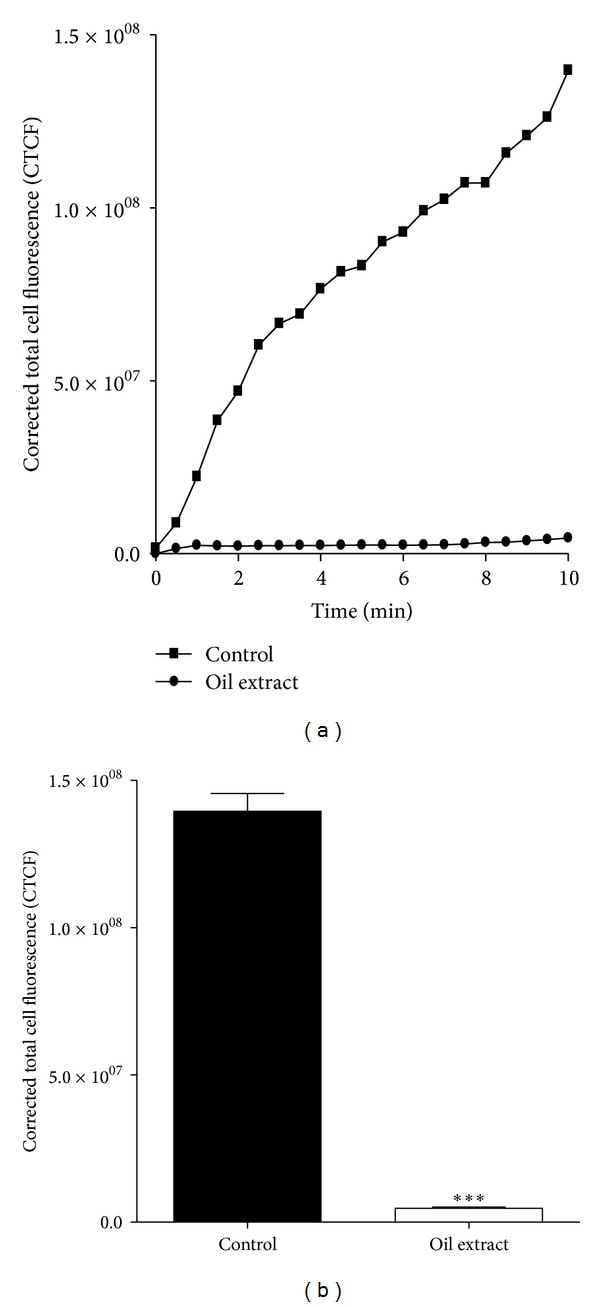
The effects of oil extract on platelet adhesion/aggregation to immobilized collagen under arterial flow conditions are shown in Figure 4. After perfusion of citrate-anticoagulated blood over collagen coated plaque surfaces at 37°C with a wall shear rate of 1000 s−1 for 10 min, rapid platelet adhesion and aggregate formation were observed (Figure 4). Oil extract (1 mg/mL) reduced collagen-induced platelet adhesion and aggregate formation under controlled flow by 91 ± 2% (P < 0.001) (Figure 4). Meanwhile, PGE1 (0.02 mmol/L) presented an inhibition by 81 ± 4% (P < 0.001).
To study if the inhibition of platelet function by oil extract is thought to be mediated by the stimulation of adenylate cyclase with increased intraplatelet cAMP concentrations, we incubated platelet with SQ22536 200 and 400 µmol/L (an adenylate cyclase inhibitor) and then with oil extract for platelet aggregation induced by ADP. We found that SQ22536 could not reverse the inhibitory effect of oil extract on platelet aggregation induced by ADP (P > NS). As control, SQ22536 400 µmol/L alone did not exert any effect on ADP (8 µmol/L)-induced platelet aggregation.
3.6. Oil Extract Reduces Platelet Spreading on Immobilized Collagen under Flow Conditions
The effects of oil extract on platelet adhesion/aggregation to immobilized collagen under arterial flow conditions are shown in Figure 4. After perfusion of citrate-anticoagulated blood over collagen coated plaque surfaces at 37°C with a wall shear rate of 1000 s−1 for 10 min, rapid platelet adhesion and aggregate formation were observed (Figure 4). Oil extract (1 mg/mL) reduced collagen-induced platelet adhesion and aggregate formation under controlled flow by 91 ± 2% (P < 0.001) (Figure 4). Meanwhile, PGE1 (0.02 mmol/L) presented an inhibition by 81 ± 4% (P < 0.001).
Figure 4Effects of oil extract on collagen-induced platelet adhesion and aggregation under arterial flow conditions. Citrate-anticoagulated blood was preincubated with DMSO 0.3% (control) or oil extract (1 mg/mL) for 15 min and then was perfused over plaque-coated surfaces for 10 min at room temperature at a shear rate of 1000 s−1. (a) shows the intensity (CTCF) over a time lapse. (b) Bar diagram (values are mean ± SEM; n = 3). ***P < 0.001. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
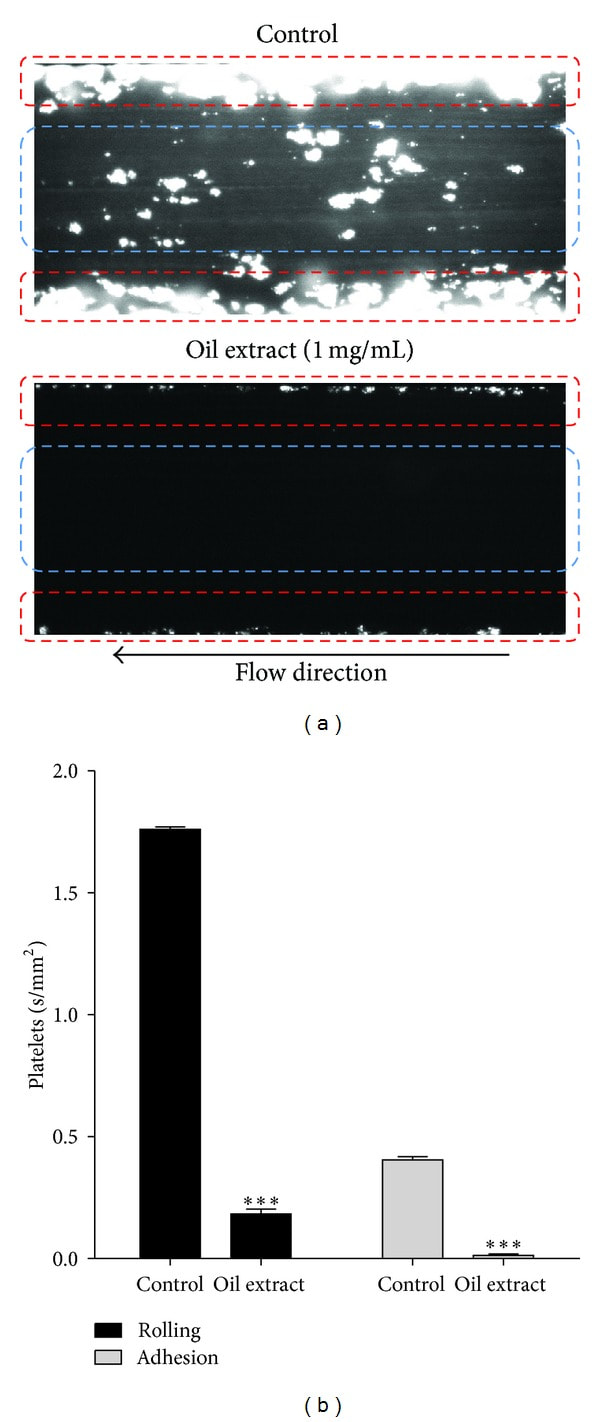
3.7. Oil Extract Reduces Platelet Rolling and Firm Adhesion
Oil extract attenuated interactions between platelet and collagen at both rolling and firm adhesion levels (Figure 5). Thus, platelet rolling in the presence of oil extract (1 mg/mL) was inhibited from 1.76 ± 0.2 in the control group to 0.2 ± 0.1 mm2/s (P < 0.001). Similarly, in the presence of oil extract (1 mg/mL), platelet firm adhesion on the collagen surface was significantly lower than that on control.
Oil extract attenuated interactions between platelet and collagen at both rolling and firm adhesion levels (Figure 5). Thus, platelet rolling in the presence of oil extract (1 mg/mL) was inhibited from 1.76 ± 0.2 in the control group to 0.2 ± 0.1 mm2/s (P < 0.001). Similarly, in the presence of oil extract (1 mg/mL), platelet firm adhesion on the collagen surface was significantly lower than that on control.
Figure 5
Effect of oil extract on platelet rolling and firm adhesion on collagen under flow conditions. (a) Representative video image of oil extract on platelets rolling and firm adhesion: platelet rolling identified by blue circles and firm adhesion by red circles. (b) Oil extract (1 mg/mL) inhibited platelets rolling and firm adhesion on collagen. Results were expressed as mean ± SEM of n = 3. ***P < 0.001. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
Effect of oil extract on platelet rolling and firm adhesion on collagen under flow conditions. (a) Representative video image of oil extract on platelets rolling and firm adhesion: platelet rolling identified by blue circles and firm adhesion by red circles. (b) Oil extract (1 mg/mL) inhibited platelets rolling and firm adhesion on collagen. Results were expressed as mean ± SEM of n = 3. ***P < 0.001. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
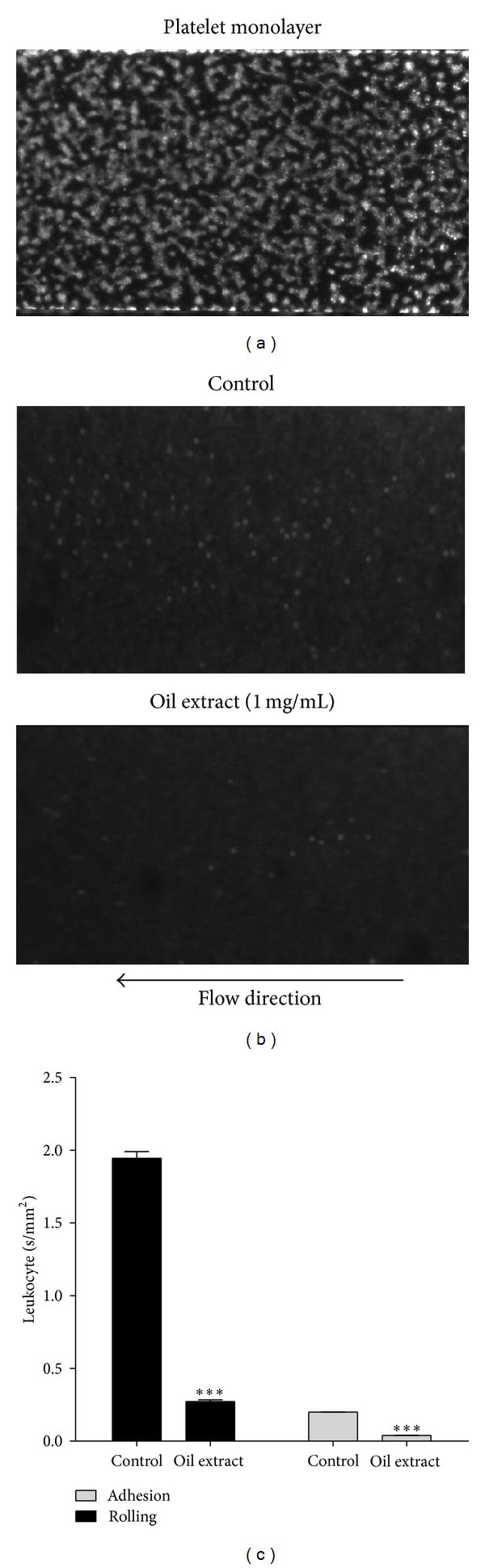
3.8. Effect of Oil Extract on Platelet-Leukocyte Interactions
Results of the real-time analysis of oil extract effects on platelet-leukocyte interactions are shown in Figure 6. Under a shear stress of 150 s−1, leukocytes rolled and got attached to activated platelet but not to collagen surfaces (data not shown). Oil extract attenuated interactions between leukocytes and platelet surface, reflecting a reduction of rolling and less adhesion (Figure 6(b)). As shown in Figure 6(c), rolling velocity of leukocytes under a shear stress of 150 s−1 over immobilized activated platelets was diminished in the presence of oil extract (1 mg/mL) from 1.9 ± 0.2 in the control group to 0.26 ± 0.1 mm2/s (P < 0.001). Similarly, oil extract resulted in lower leukocyte firm adhesion on the surface of the platelet monolayer as compared to the control group (P < 0.001) (Figure 6(c)).
Results of the real-time analysis of oil extract effects on platelet-leukocyte interactions are shown in Figure 6. Under a shear stress of 150 s−1, leukocytes rolled and got attached to activated platelet but not to collagen surfaces (data not shown). Oil extract attenuated interactions between leukocytes and platelet surface, reflecting a reduction of rolling and less adhesion (Figure 6(b)). As shown in Figure 6(c), rolling velocity of leukocytes under a shear stress of 150 s−1 over immobilized activated platelets was diminished in the presence of oil extract (1 mg/mL) from 1.9 ± 0.2 in the control group to 0.26 ± 0.1 mm2/s (P < 0.001). Similarly, oil extract resulted in lower leukocyte firm adhesion on the surface of the platelet monolayer as compared to the control group (P < 0.001) (Figure 6(c)).
Figure 6
Oil extract reduces leukocyte rolling and firm adhesion over collagen-bound platelet monolayers. (a) Homogeneous carpet of spread platelets labeled with calcein-AM formed on the collage. (b) Representative images of rhodamine 6G labeled leukocyte rolling and adhesion on platelet monolayer at shear rate of 150 s−1 in absence (DMSO 0.3%) or presence of oil extract (1 mg/mL). (c) Velocities of leukocyte rolling and firm adhesion on platelet monolayer surface. Results were expressed as mean ± SEM of n = 3. ***P < 0.001. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
Oil extract reduces leukocyte rolling and firm adhesion over collagen-bound platelet monolayers. (a) Homogeneous carpet of spread platelets labeled with calcein-AM formed on the collage. (b) Representative images of rhodamine 6G labeled leukocyte rolling and adhesion on platelet monolayer at shear rate of 150 s−1 in absence (DMSO 0.3%) or presence of oil extract (1 mg/mL). (c) Velocities of leukocyte rolling and firm adhesion on platelet monolayer surface. Results were expressed as mean ± SEM of n = 3. ***P < 0.001. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
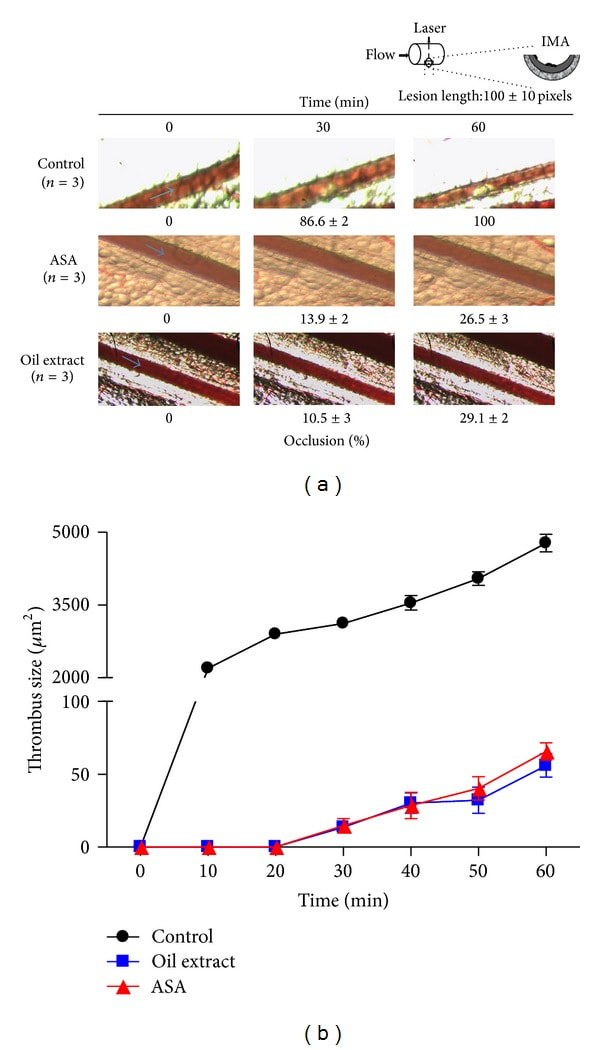
3.9. Effect of Oil Extract on Arterial Thrombus Formation In Vivo
To examine the in vivo antithrombotic activity of oil extract, we evaluated the effects of oil extract on laser-injured thrombus formation in mice mesenteric artery in vivo. As shown in Figure 7, in untreated mice (control), mesenteric artery was totally blocked by a stable bulky thrombus at 40 min. In contrast, further analysis revealed that the time to form the artery thrombosis (>2500 µm2) was drastically prolonged in oil extract-treated mice compared to the mice receiving the same volume of vehicle (Figure 7). Thus, one intraperitoneally bolus injection of oil extract (200 mg/kg) 30 min before laser injury prevented thrombus formation over 60 min after laser injury by 71 ± 2% (P < 0.001). Currently ASA is the gold standard for secondary prevention of stroke of vascular origin; therefore, we compared the antithrombotic role of oil extract with ASA. Under our conditions both oil extract (200 mg/kg) and ASA (200 mg/kg) exhibiting the same antithrombotic efficacy (Figure 7), especially when vessel occlusion is evaluated.
To examine the in vivo antithrombotic activity of oil extract, we evaluated the effects of oil extract on laser-injured thrombus formation in mice mesenteric artery in vivo. As shown in Figure 7, in untreated mice (control), mesenteric artery was totally blocked by a stable bulky thrombus at 40 min. In contrast, further analysis revealed that the time to form the artery thrombosis (>2500 µm2) was drastically prolonged in oil extract-treated mice compared to the mice receiving the same volume of vehicle (Figure 7). Thus, one intraperitoneally bolus injection of oil extract (200 mg/kg) 30 min before laser injury prevented thrombus formation over 60 min after laser injury by 71 ± 2% (P < 0.001). Currently ASA is the gold standard for secondary prevention of stroke of vascular origin; therefore, we compared the antithrombotic role of oil extract with ASA. Under our conditions both oil extract (200 mg/kg) and ASA (200 mg/kg) exhibiting the same antithrombotic efficacy (Figure 7), especially when vessel occlusion is evaluated.
Figure 7
Oil extract inhibits laser-induced thrombus formation in mesenteric artery of C57BL/6 mice. (a) Representative images of thrombus formation at baseline, 10, 20, 30, 40, 50, and 60 min after laser-injured vascular injury in mouse mesenteric artery. (b) Time course changes of thrombus growth rate among the control (DMSO 0.3%), ASA (acetylsalicylic acid), and oil extract for 60 min after laser irradiation. The graph depicts the means ± SEM of n = 3 experiments. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
Oil extract inhibits laser-induced thrombus formation in mesenteric artery of C57BL/6 mice. (a) Representative images of thrombus formation at baseline, 10, 20, 30, 40, 50, and 60 min after laser-injured vascular injury in mouse mesenteric artery. (b) Time course changes of thrombus growth rate among the control (DMSO 0.3%), ASA (acetylsalicylic acid), and oil extract for 60 min after laser irradiation. The graph depicts the means ± SEM of n = 3 experiments. We eliminated the background (yellow colored oil) with plugin that implemented ImageJ's (subtract background command) to avoid quenching phenomena for the yellow colored oil (the Amazonian oil).
4. DISCUsSION
In the present study we have demonstrated for the first time that oil extract of fruit from Mauritia flexuosa possesses antiplatelet activity and inhibits in vivo thrombus formation. In this way, many studies have provided evidence of the protective role of healthy diets in the prevention of CVD . More specifically, a number of dietary components including some fats, polyphenols, and nucleosides have shown to diminish platelet activation [21, 22]. To this respect, our group has recently isolated and identified adenosine and guanosine as a bioactive compound in Solanum lycopersicum (a cherry type tomato) with potent antiplatelet activity .
Fruit from the palm Mauritia flexuosa for its high nutritional value (fat 38%, fiber 30%, carbohydrate 28%, and protein 5%) is a fundamental part of the human diet. Its fruit also contains vitamin A, potassium, calcium, iron, magnesium, and the highest carotenoid contents . In Venezuela indigenous people use this fruit as bread , and in Brazil and Ecuador during times of flooding it is the biggest source of food. Currently, fruits of the palm Mauritia flexuosa are one of the Amazon fruits more commercialized (pulp, soda, lollipops, ice cream, jams, and yoghurt) . Limonene is a main component of citrus essential oils which are commonly extracted from their matrix by using distillation and it plays an important role in the field of flavours and fragrances for many years thanks to its physical and chemical properties .
In the past 2 decades, views about dietary unsaturated fatty acids have moved from speculation about their functions to solid evidence that they not only are essential nutrients but also may favorably modulate many diseases . Thus changes in dietary fatty acids may have mild, but significant, effects on eicosanoid production, platelet aggregation, and blood pressure . Fatty acid determination by gas chromatography in the walnut of fruits from the palm Mauritia flexuosa evidenced the presence of unsaturated fatty acids: oleic (27%) and linoleic (22.6%) (data not shown) . Therefore, according to this study, the peel and walnut have a high content of oleic acid.
With respect to antiplatelet and antithrombotic activities, we firstly analyzed the potential antiplatelet properties of the oil extract. The oil extract inhibited platelet secretion and aggregation elicited by a broad range of agonists. Furthermore, we observed that oil extract prevents platelet rolling and firm adhesion onto collagen under flow conditions.
Thrombin-induced sP-selectin release from human platelets does not seem to be the target pathway of the oil of interest. In addition, platelets are activated by different activators via complex signal pathways. Therefore, we speculate that oil extract may block a common signal pathway involved in platelet activation without participation of adenylyl cyclase pathway (studied by SQ22536). These healthy activities of oil extract from peels of Mauritia flexuosa fruits that prevent CVD are mainly due to the presence of unsaturated fatty acids (oleic, linoleic, and palmitoleic acids).
In this sense, it has been reported that PPARs agonists (unsaturated fatty acids), which involve an elevation of cAMP, involve subsequent inhibition of platelet function against various agonists . In this way, oleic acid is capable of affecting platelet function . Oleic acid is a potent inhibitor of phospholipase A2 which would explain its generally low activity in human platelet extracts and its marked increase of activity during the course of enzyme purification . In addition, linoleic acid present in both the peel and walnut from Mauritia flexuosa was shown to inhibit the formation of arterial thrombosis, the expression of tissue factor, and platelet aggregation . These properties are directly related to the prevention of thrombi development occurring in stroke.
Taking into consideration all of these findings, we further provided in vivo evidence of such in vitro-related oil extract antithrombotic effects. Indeed, using a murine model of real-time thrombus formation, we demonstrate that oil extract inhibited arterial thrombus growth at the same concentration of aspirin, a widely used antiplatelet agent. We even demonstrate, that oil extract administration diminished thrombus growth with kinetics of thrombus inhibition similar to that of aspirin.
The broad range of antiplatelet and antithrombotic effects found in the oil extracted from Mauritia flexuosa may render this functionally active principle in potent inhibitors of platelet function with a potential preventive effect on thrombus formation.
Fruit from the palm Mauritia flexuosa for its high nutritional value (fat 38%, fiber 30%, carbohydrate 28%, and protein 5%) is a fundamental part of the human diet. Its fruit also contains vitamin A, potassium, calcium, iron, magnesium, and the highest carotenoid contents . In Venezuela indigenous people use this fruit as bread , and in Brazil and Ecuador during times of flooding it is the biggest source of food. Currently, fruits of the palm Mauritia flexuosa are one of the Amazon fruits more commercialized (pulp, soda, lollipops, ice cream, jams, and yoghurt) . Limonene is a main component of citrus essential oils which are commonly extracted from their matrix by using distillation and it plays an important role in the field of flavours and fragrances for many years thanks to its physical and chemical properties .
In the past 2 decades, views about dietary unsaturated fatty acids have moved from speculation about their functions to solid evidence that they not only are essential nutrients but also may favorably modulate many diseases . Thus changes in dietary fatty acids may have mild, but significant, effects on eicosanoid production, platelet aggregation, and blood pressure . Fatty acid determination by gas chromatography in the walnut of fruits from the palm Mauritia flexuosa evidenced the presence of unsaturated fatty acids: oleic (27%) and linoleic (22.6%) (data not shown) . Therefore, according to this study, the peel and walnut have a high content of oleic acid.
With respect to antiplatelet and antithrombotic activities, we firstly analyzed the potential antiplatelet properties of the oil extract. The oil extract inhibited platelet secretion and aggregation elicited by a broad range of agonists. Furthermore, we observed that oil extract prevents platelet rolling and firm adhesion onto collagen under flow conditions.
Thrombin-induced sP-selectin release from human platelets does not seem to be the target pathway of the oil of interest. In addition, platelets are activated by different activators via complex signal pathways. Therefore, we speculate that oil extract may block a common signal pathway involved in platelet activation without participation of adenylyl cyclase pathway (studied by SQ22536). These healthy activities of oil extract from peels of Mauritia flexuosa fruits that prevent CVD are mainly due to the presence of unsaturated fatty acids (oleic, linoleic, and palmitoleic acids).
In this sense, it has been reported that PPARs agonists (unsaturated fatty acids), which involve an elevation of cAMP, involve subsequent inhibition of platelet function against various agonists . In this way, oleic acid is capable of affecting platelet function . Oleic acid is a potent inhibitor of phospholipase A2 which would explain its generally low activity in human platelet extracts and its marked increase of activity during the course of enzyme purification . In addition, linoleic acid present in both the peel and walnut from Mauritia flexuosa was shown to inhibit the formation of arterial thrombosis, the expression of tissue factor, and platelet aggregation . These properties are directly related to the prevention of thrombi development occurring in stroke.
Taking into consideration all of these findings, we further provided in vivo evidence of such in vitro-related oil extract antithrombotic effects. Indeed, using a murine model of real-time thrombus formation, we demonstrate that oil extract inhibited arterial thrombus growth at the same concentration of aspirin, a widely used antiplatelet agent. We even demonstrate, that oil extract administration diminished thrombus growth with kinetics of thrombus inhibition similar to that of aspirin.
The broad range of antiplatelet and antithrombotic effects found in the oil extracted from Mauritia flexuosa may render this functionally active principle in potent inhibitors of platelet function with a potential preventive effect on thrombus formation.
5. conclusion
The data presented herein demonstrate, for the first time to our knowledge, the protective effect of oil extract from Mauritia flexuosa on platelet activation and thrombosis formation. These effects, in terms of primary prevention, could modify cardiovascular risk without any of the side effects normally associated with antiplatelet drugs. Moreover, Mauritia flexuosa may constitute a functional ingredient adding antiplatelet activities to processed foods.
Conflict of Interests
The authors report no conflict of interests.
Acknowledgments
This work was funded by the CONICYT-Regional, gore Maule, CEAP, Interdisciplinary Excellence Research Program on Healthy Aging (PIEI-ES), and it was supported by Grant no. 1130216 (I.P., M.G., R.M., M.A., J.C.) from Fondecyt, Chile.
Conflict of Interests
The authors report no conflict of interests.
Acknowledgments
This work was funded by the CONICYT-Regional, gore Maule, CEAP, Interdisciplinary Excellence Research Program on Healthy Aging (PIEI-ES), and it was supported by Grant no. 1130216 (I.P., M.G., R.M., M.A., J.C.) from Fondecyt, Chile.
references
1. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (second of two parts) The New England Journal of Medicine. 1992;326(5):310–318. [PubMed] [Google Scholar]
2. Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nature Reviews Drug Discovery. 2010;9(2):154–169. [PubMed] [Google Scholar]
3. Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. [PMC free article] [PubMed] [Google Scholar]
4. Palomo I, Leiva E, Vásquez M. Dieta Mediterranea: Prevención de las Enfermedades Cardiovasculares. Talca, Chile: Universidad de Talca Press; 2007. [Google Scholar]
5. Fuentes E, Carle R, Astudillo L, et al. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9 pages.867578 [PMC free article] [PubMed] [Google Scholar]
6. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England Journal of Medicine. 2013;368:1279–1290. [PubMed] [Google Scholar]
7. Bemelmans WJ, Lefrandt JD, Feskens EJ, et al. Increased α-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. European Journal of Clinical Nutrition. 2004;58(7):1083–1089. [PubMed] [Google Scholar]
8. Covas M-I, Nyyssönen K, Poulsen HE, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Annals of Internal Medicine. 2006;145(5):333–341. [PubMed] [Google Scholar]
9. Massaro M, Scoditti E, Carluccio M, Montinari M, de Caterina R. Omega-3 fatty acids, inflammation and angiogenesis: nutrigenomic effects as an explanation for anti-atherogenic and anti-inflammatory effects of fish and fish oils. Journal of Nutrigenetics and Nutrigenomics. 2007;1(1-2):4–23. [PubMed] [Google Scholar]
10. Gilbert B. Economic plants of the Amazon. In: Seidl P, Gottlieb OR, Kaplan MA, editors. Chemistry of the Amazon: Biodiversity, Natural Products and Environmental Issues. Washington, DC, USA: ACS; 1995. pp. 9–33. (Symposium Series). [Google Scholar]
11. Gilmore MP, Endress BA, Horn CM. The socio-cultural importance of Mauritia flexuosa palm swamps (aguajales) and implications for multi-use management in two Maijuna communities of the Peruvian Amazon. Journal of Ethnobiology and Ethnomedicine. 2013;9, article 29 [PMC free article] [PubMed] [Google Scholar]
12. Albuquerque MLS, Guedes I, Alcantara P, Jr., et al. Characterization of Buriti (Mauritia flexuosa L.) oil by absorption and emission spectroscopies. Journal of the Brazilian Chemical Society. 2005;16(6):1113–1117. [Google Scholar]
13. Franc LF, Reber G, Meireles MAA, Machado NT, Brunner G. Supercritical extraction of carotenoids and lipids from buriti (Mauritia flexuosa), a fruit from the Amazon region. Journal of Supercritical Fluids. 1999;14(3):247–256. [Google Scholar]
14. Zanatta CF, Mitjans M, Urgatondo V, Rocha-Filho PA, Vinardell MP. Photoprotective potential of emulsions formulated with Buriti oil (Mauritia flexuosa) against UV irradiation on keratinocytes and fibroblasts cell lines. Food and Chemical Toxicology. 2010;48(1):70–75. [PubMed] [Google Scholar]
15. Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—methanol. Journal of Lipid Research. 1964;5:600–608. [PubMed] [Google Scholar]
16. Born GV, Cross MJ. The aggregation of blood platelets. Journal of Physiology. 1963;168:178–195. [PMC free article] [PubMed] [Google Scholar]
17. Conant CG, Schwartz MA, Nevill T, Ionescu-Zanetti C. Platelet adhesion and aggregation under flow using microfluidic flow cells. Journal of Visualized Experiments. 2009;(32)e1644 [PMC free article] [PubMed] [Google Scholar]
18. Appeldoorn CC, Bonnefoy A, Lutters BC, et al. Gallic acid antagonizes P-selectin-mediated platelet-leukocyte interactions: implications for the French paradox. Circulation. 2005;111(1):106–112. [PubMed] [Google Scholar]
19. Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. The American Journal of Clinical Nutrition. 2003;78:544S–551S. [PubMed] [Google Scholar]
20. Fuentes EJ, Astudillo LA, Gutiérrez MI, et al. Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood Coagulation and Fibrinolysis. 2012;23(2):109–117. [PubMed] [Google Scholar]
21. Fuentes E, Castro R, Astudillo L, et al. Bioassay-guided isolation and HPLC determination of bioactive compound that relate to the antiplatelet activity (adhesion, secretion, and aggregation) from Solanum lycopersicum . Evidence-Based Complementary and Alternative Medicine. 2012;2012:10 pages.147031 [PMC free article] [PubMed] [Google Scholar]
22. Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. Journal of Thrombosis and Haemostasis. 2004;2(12):2138–2145. [PubMed] [Google Scholar]
23. Fuentes E, Alarcon M, Astudillo L, et al. Protective mechanisms of guanosine from Solanum lycopersicum on agonist-induced platelet activation: role of sCD40L. Molecules. 2013;18:8120–8135. [PMC free article] [PubMed] [Google Scholar]
24. Galeano G. Las Palmas de la Region Araracuara. Bogotá, Colombia: TROPENBOS. Instituto de Ciencias Naturales. Universidad nacional de Colombia; 1992. [Google Scholar]
25. Ferreira BS, de Almeida CG, Faza LP, et al. Comparative properties of amazonian oils obtained by different extraction methods. Molecules. 2011;16(7):5875–5885. [PMC free article] [PubMed] [Google Scholar]
26. Braun A. Cultivated palms of Venezuela. Principes. 1968;12:2–4. [Google Scholar]
27. Hiraoka M. Miriti (Mauritia flexuosa) palms and their uses and management among the ribeirinhos of the Amazon estuary. In: Padoch C, Ayres JM, Pinedo-Vasquez M, Henderson A, editors. Changes in Soil Formation and Vegetation on Silt Bars and Backslopes of Levees following Intensive Production of Rice and Jute. New York, NY, USA: The New York Botanical Garden Press; 1999. pp. 169–186. [Google Scholar]
28. Agra MF, Freitas PF, Barbosa-Filho JM. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Revista Brasileira de Farmacognosia. 2007;17:17114–17140. [Google Scholar]
29. Mira B, Blasco M, Berna A, Subirats S. Supercritical CO2 extraction of essential oil from orange peel. Effect of operation conditions on the extract composition. Journal of Supercritical Fluids. 1999;14(2):95–104. [Google Scholar]
30. Selli S, Cabaroglu T, Canbas A. Volatile flavour components of orange juice obtained from the cv. Kozan of Turkey. Journal of Food Composition and Analysis. 2004;17(6):789–796. [Google Scholar]
31. Connor WE. Importance of n-3 fatty acids in health and disease. The American Journal of Clinical Nutrition. 2000;71:171S–175S. [PubMed] [Google Scholar]
32. Lahoz C, Alonso R, Ordovás JM, López-Farré A, de Oya M, Mata P. Effects of dietary fat saturation on eicosanoid production, platelet aggregation and blood pressure. European Journal of Clinical Investigation. 1997;27(9):780–787. [PubMed] [Google Scholar]
33. Sirtori CR, Tremoli E, Gatti E, et al. Controlled evaluation of fat intake in the Mediterranean diet: comparative activities of olive oil and corn oil on plasma lipids and platelets in high-risk patients. The American Journal of Clinical Nutrition. 1986;44(5):635–642. [PubMed] [Google Scholar]
34. Rodríguez-Pérez W. Estudio comparativo de la composición de ácidos grasos del aceite de semilla de plantas de la Amazonia Colombiana. Momentos de Ciencia. 2005;2:75–81. [Google Scholar]
35. Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARα . Journal of Lipid Research. 1999;40(8):1426–1433. [PubMed] [Google Scholar]
36. Chou T-C, Shih C-Y, Chen Y-T. Inhibitory effect of α-Lipoic acid on platelet aggregation is mediated by PPARs. Journal of Agricultural and Food Chemistry. 2011;59(7):3050–3059. [PubMed] [Google Scholar]
37. Yokoi H, Mizukami H, Nagatsu A, Tanabe H, Inoue M. Hydroxy monounsaturated fatty acids as agonists for peroxisome proliferator-activated receptors. Biological and Pharmaceutical Bulletin. 2010;33(5):854–861. [PubMed] [Google Scholar]
38. Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Olive oil and haemostasis: platelet function, thrombogenesis and fibrinolysis. Current Pharmaceutical Design. 2011;17(8):778–785. [PubMed] [Google Scholar]
39. Ballou LR, Cheung WY. Inhibition of human platelet phospholipase A2 activity by unsaturated fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(2):371–375. [PMC free article] [PubMed] [Google Scholar]
Articles from Evidence-based Complementary and Alternative Medicine : eCAM are provided here courtesy of Hindawi Limited