SANGRE DE GRADO
INTRODUCTION
Croton lechleri Muell. Arg. (1974), Class Dicot, Order Euphorbiales, Family Euphorbiaceae. This species is primarily used because of its healing properties in the treatment of gastric ulcers, puerperium, tonsillitis, pharyngitis, tumors, for birth control, and others. The use of sangre de grado came from the 1600s so it is widely distributed and is being continued up to day.
SCIENTIFIC NAME
Croton lechleri Muell. Arg. (1974)
SYNONYM
Croton palanostigma Klotrch (1951) - C. tarapotensis Muell. Arg. (1951) - C. draconoides Muell. Arg.
COMMON NAMES
Sangre de grado, dragon's blood, stick drago, dragon blood - Irare, jimi mosho, shawan karo: Shipibo-Conibo - Pocure, racurana, uksavakiro, widnku: Amarakaeri
PLANT MORPHOLOGY
Habit: tree 10-15 m tall, with a broad and rounded crown. Stem: the trunk with whitish bark and glabrous, which when cut secretes a vinous red latex which is used in the pharmaceutical industry. The branches are covered with stellate hairs.
Leaves: cordate to ovate, 10-15 cm long and 7-11 cm wide, apex acuminate, margin entire, glabrous, with 2 glands at the base of the leaf, penninervia. Inflorescence and flowers: flowers arranged in loose clusters terminal, with the male flowers located at the top of the inflorescence and female flowers at the bottom. Calyx with 5 granulated sepals, corolla with 5 elliptic petals, the pistil with bifid stigma, superior ovary; stamens 15-18 cm long, with pubescent filaments.
Croton lechleri Muell. Arg. (1974), Class Dicot, Order Euphorbiales, Family Euphorbiaceae. This species is primarily used because of its healing properties in the treatment of gastric ulcers, puerperium, tonsillitis, pharyngitis, tumors, for birth control, and others. The use of sangre de grado came from the 1600s so it is widely distributed and is being continued up to day.
SCIENTIFIC NAME
Croton lechleri Muell. Arg. (1974)
SYNONYM
Croton palanostigma Klotrch (1951) - C. tarapotensis Muell. Arg. (1951) - C. draconoides Muell. Arg.
COMMON NAMES
Sangre de grado, dragon's blood, stick drago, dragon blood - Irare, jimi mosho, shawan karo: Shipibo-Conibo - Pocure, racurana, uksavakiro, widnku: Amarakaeri
PLANT MORPHOLOGY
Habit: tree 10-15 m tall, with a broad and rounded crown. Stem: the trunk with whitish bark and glabrous, which when cut secretes a vinous red latex which is used in the pharmaceutical industry. The branches are covered with stellate hairs.
Leaves: cordate to ovate, 10-15 cm long and 7-11 cm wide, apex acuminate, margin entire, glabrous, with 2 glands at the base of the leaf, penninervia. Inflorescence and flowers: flowers arranged in loose clusters terminal, with the male flowers located at the top of the inflorescence and female flowers at the bottom. Calyx with 5 granulated sepals, corolla with 5 elliptic petals, the pistil with bifid stigma, superior ovary; stamens 15-18 cm long, with pubescent filaments.
Fruit: pubescent capsule 5 mm in length and 4-6 mm wide.
Habitat: this species is found in both high and low jungle in Peru and Ecuador, below 1000 m. In Peru is in Departments of Amazonas, Cuzco, Huanuco, Loreto, Madre de Dios and San Martin.
ETHNOMEDICINE
Over the years, dragon's blood has been used to heal wounds mainly, but anti-inflammatory, antiseptic and hemostatic properties have also been reported.
Other uses include: treatment of diarrhea, gastrointestinal ulcers, pyorrhea, menstrual cramps, fevers from digestive causes, vaginal baths before delivery, for bleeding after childbirth, urinary retention (when taken in small doses), and skin conditions. In addition, anticancer action is attributed to C. lechleri. About 8 drops are administered in all these uses of folk medicine – although there are doses which may reach up to 20 or 30 drops – and are usually added to an infusion of any aromatic plant.
CHEMICAL CONSTITUENTS
The phytochemical characterization of the sap of dragon’s blood has led to the finding that the oligomeric proanthocyanidins (catechin, epicatechin, gallocatechin, epigallocatechin at a different degree of polymerization constitute almost 90% of its dry weight; among them are dimeric procyanidins B-1 and B-4, dimers and trimers as catechin-(4α→8)- epigallocatechin, gallocatechin- (4α→8)-epicatechin, gallocatechin-(4α→6)-epigallocatechin, catechin-(4α→8)-gallocatechin-(4α→8)-gallocatechin and gallocatechin-(4α→8)-gallocatechin-(4α→8)-epigallocatechin and other higher oligomers.
SP-303. a mixture of basic monomers obtained from the sap of dragon’s blood, consists mainly of (+)- gallocatechin and (-)-galloepicatechin and to a lesser amount, of (+)-catechin and (-)-epicatechin. Various minor compounds have also been found: one is the alkaloid taspine found in the sap of mature tree.
Others are the dihydrobenzofuran lignans 3’,4-O-dimethylcedrusin and 4-Omethylcedrusin, 1,3,5-trimethoxybenzene and 2,4,6,- trimethoxyphenol, various diterpenoids clerodane type: korberin A and B and norisoprenoids blumenols B and C, 4,5-dihydroblumenol A and floribundic acid glucoside. Clerodanes as crolechinol and crolechinic acid have been also isolated from the bark.
Habitat: this species is found in both high and low jungle in Peru and Ecuador, below 1000 m. In Peru is in Departments of Amazonas, Cuzco, Huanuco, Loreto, Madre de Dios and San Martin.
ETHNOMEDICINE
Over the years, dragon's blood has been used to heal wounds mainly, but anti-inflammatory, antiseptic and hemostatic properties have also been reported.
Other uses include: treatment of diarrhea, gastrointestinal ulcers, pyorrhea, menstrual cramps, fevers from digestive causes, vaginal baths before delivery, for bleeding after childbirth, urinary retention (when taken in small doses), and skin conditions. In addition, anticancer action is attributed to C. lechleri. About 8 drops are administered in all these uses of folk medicine – although there are doses which may reach up to 20 or 30 drops – and are usually added to an infusion of any aromatic plant.
CHEMICAL CONSTITUENTS
The phytochemical characterization of the sap of dragon’s blood has led to the finding that the oligomeric proanthocyanidins (catechin, epicatechin, gallocatechin, epigallocatechin at a different degree of polymerization constitute almost 90% of its dry weight; among them are dimeric procyanidins B-1 and B-4, dimers and trimers as catechin-(4α→8)- epigallocatechin, gallocatechin- (4α→8)-epicatechin, gallocatechin-(4α→6)-epigallocatechin, catechin-(4α→8)-gallocatechin-(4α→8)-gallocatechin and gallocatechin-(4α→8)-gallocatechin-(4α→8)-epigallocatechin and other higher oligomers.
SP-303. a mixture of basic monomers obtained from the sap of dragon’s blood, consists mainly of (+)- gallocatechin and (-)-galloepicatechin and to a lesser amount, of (+)-catechin and (-)-epicatechin. Various minor compounds have also been found: one is the alkaloid taspine found in the sap of mature tree.
Others are the dihydrobenzofuran lignans 3’,4-O-dimethylcedrusin and 4-Omethylcedrusin, 1,3,5-trimethoxybenzene and 2,4,6,- trimethoxyphenol, various diterpenoids clerodane type: korberin A and B and norisoprenoids blumenols B and C, 4,5-dihydroblumenol A and floribundic acid glucoside. Clerodanes as crolechinol and crolechinic acid have been also isolated from the bark.
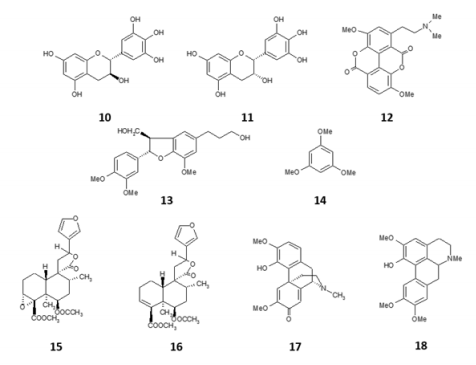
Figure 2: Some chemical compounds isolated from Croton lechleri.
Ethyl propianate, as well as 2-methyl butanol, 3-methyl-2-pentanol and eucalyptol have been found as volatile components of the latex.
The analyses of the essential oil from stem bark of C. lechleri from Amazonian Ecuador demonstrated a remarkable sesquiterpene prevail characterized, in order of abundance, by sesquicineole, αcalacorene, 1,10-di-epi-cubenol, β-calacorene and epi-cedrol; other minor components are the monoterpenes limonene, borneol and pcymene.
The leaves also contain taspine and the alkaloid sinoacutine known as morphinan-7-one; as well as the aporphines magnoflorine, isoboldine, norisoboldine, glaucine, and thaliporphine.
Flavonoids, mainly rutin and vitexin, were also reported (Figure 2).
PHARMACOLOGY
A bioassay-guided fractionation study of C. lechleri (CL) sap showed that the n-BuOH fraction had the highest antioxidant activity in the DPPH test. The antioxidant activity is explained by the high concentration of phenolic compounds, especially epigallocatechin. This compound had even a lower IC50 than the controls ascorbic acid, quercetin and trolox.
According to Desmarchelier et al. and Risco et al., the sap of CL can act as an antioxidant or a prooxidant, depending on the concentration used in several in vivo assays. CL sap showed good activity against Bacillus subtilis and Escherechia coli.
The compounds accounted responsible for these activities were 1,3,5-trimethoxybencene; 2,4,6- trimethoxyphenol and korberins A and B.
Itokawa et al. performed a bioassay-guided fractionation of the sap of CP (Croton palanostigma) and isolated taspine as the main cytotoxic compound against KB and V-79 cells [49a].
Taspine is also cytotoxic against melanoma SK23 and colon cancer HT29 cells.
A methanol extract of Croton lechleri leaves is cytotoxic against HeLa cells, without being toxic to normal human cells. It also showed anticancer effect in vivo by inhibiting tumor growth in mice with HeLa tumor [46].
Fayad et al. demonstrated that the alkaloid taspine inhibits both topoisomerases I and II in cells overexpressing drug efflux transporters [49c].
In the Ames/Salmonella test, the CL sap showed no mutagenic activity on the Salmonella typhimurium strains T98 and T10 [50a]; however, it was mutagenic against strain TA1535 in the presence of metabolic activation [50b]. In an in vitro model, the sap of CL behaved as a potent inhibitor of cutaneous neurogenic inflammation by suppressing the release of substance P by sensory afferent nerves [51a,b].
Topical administration of sangre de grado balm reduced rat paw edema and caused relief of itching, pain and redness caused by insect bites in a small group of pest control workers [51b].
Risco et al. evaluated the antiinflammatory activity of taspine and the sap of SG. The sap was almost as active as naproxen in rat paw edema caused by carragenin [52a]. Taspine (20 mg/Kg) was equivalent to indomethacin (1 mg/Kg) in an in vivo adjuvant polyarthritis model [52b].
The analyses of the essential oil from stem bark of C. lechleri from Amazonian Ecuador demonstrated a remarkable sesquiterpene prevail characterized, in order of abundance, by sesquicineole, αcalacorene, 1,10-di-epi-cubenol, β-calacorene and epi-cedrol; other minor components are the monoterpenes limonene, borneol and pcymene.
The leaves also contain taspine and the alkaloid sinoacutine known as morphinan-7-one; as well as the aporphines magnoflorine, isoboldine, norisoboldine, glaucine, and thaliporphine.
Flavonoids, mainly rutin and vitexin, were also reported (Figure 2).
PHARMACOLOGY
A bioassay-guided fractionation study of C. lechleri (CL) sap showed that the n-BuOH fraction had the highest antioxidant activity in the DPPH test. The antioxidant activity is explained by the high concentration of phenolic compounds, especially epigallocatechin. This compound had even a lower IC50 than the controls ascorbic acid, quercetin and trolox.
According to Desmarchelier et al. and Risco et al., the sap of CL can act as an antioxidant or a prooxidant, depending on the concentration used in several in vivo assays. CL sap showed good activity against Bacillus subtilis and Escherechia coli.
The compounds accounted responsible for these activities were 1,3,5-trimethoxybencene; 2,4,6- trimethoxyphenol and korberins A and B.
Itokawa et al. performed a bioassay-guided fractionation of the sap of CP (Croton palanostigma) and isolated taspine as the main cytotoxic compound against KB and V-79 cells [49a].
Taspine is also cytotoxic against melanoma SK23 and colon cancer HT29 cells.
A methanol extract of Croton lechleri leaves is cytotoxic against HeLa cells, without being toxic to normal human cells. It also showed anticancer effect in vivo by inhibiting tumor growth in mice with HeLa tumor [46].
Fayad et al. demonstrated that the alkaloid taspine inhibits both topoisomerases I and II in cells overexpressing drug efflux transporters [49c].
In the Ames/Salmonella test, the CL sap showed no mutagenic activity on the Salmonella typhimurium strains T98 and T10 [50a]; however, it was mutagenic against strain TA1535 in the presence of metabolic activation [50b]. In an in vitro model, the sap of CL behaved as a potent inhibitor of cutaneous neurogenic inflammation by suppressing the release of substance P by sensory afferent nerves [51a,b].
Topical administration of sangre de grado balm reduced rat paw edema and caused relief of itching, pain and redness caused by insect bites in a small group of pest control workers [51b].
Risco et al. evaluated the antiinflammatory activity of taspine and the sap of SG. The sap was almost as active as naproxen in rat paw edema caused by carragenin [52a]. Taspine (20 mg/Kg) was equivalent to indomethacin (1 mg/Kg) in an in vivo adjuvant polyarthritis model [52b].
CL sap decreases the cellular immune response and is also a potent inhibitor of the classical and alternative complement pathways [6a].
Vaisberg et al. demonstrated that the topical application for a period of 17 months of either CL sap or taspine to the skin of rats is not tumorogenic [53a].
Using a mice model of wound healing, Vaisberg et al. found that taspine has a dose-response cicatrizant effect [53a]. The activity of taspine was greater than the sap and its mechanism of action might be the enhancement of fibroblasts migration [53a,b].
On the other hand, in an in vitro model Pieters et al. found that 3´,4- O-dimethylcedrusin stimulates the proliferation of endothelial cells. In this model, taspine was rather cytotoxic [41,54a].
In an in vivo model, the sap (rich in proanthocyanidins) stimulates wound contraction, formation of the crust and synthesis of collagen. The compound 3',4-O-dimethylcedrusin also improved wound healing, but was less active than the sap.
According to Pieters et al., taspine has no influence in the cicatrization even at high concentrations [54b].
In a rat model of gastric ulcers the oral treatment with CL reduced ulcer size, myeloperoxidase activity and bacterial content of the ulcer.
The expression of proinflammatory genes (TNF-, iNOS, IL1β, IL-6 y COX-2) was also reduced during SG treatment [55].
In vitro and in vivo studies demonstrate that SP-303, a large proanthocyanidin oligomer isolated from the sap of C. lechleri, has good activity against respiratory syncytial, influenza A and parainfluenza viruses.
These activities are comparable to ribavirin. SP-303 is also active against herpes virus type 1 and 2, as well against hepatitis virus A and [39b].
SP-303 in vivo decreases intestinal secretion caused by cholera toxin and in vitro reduces cAMP-mediated Cl- secretion [56a,b].
A double blind, placebo-controlled clinical trial in 169 patients with travelers´ diarrhoea showed that, compared to placebo, SP-303 was able to shorten the duration of the diarrhea by 21% [57a].
SP-303 was eventually named crofelemer and patented by Napo Pharmaceuticals. Crofelemer displays its intestinal antisecretory activity by inhibiting two secretory channels: the cystic fibrosis conductance regulator and calcium-activated chloride cannel [57b].
Following phase III clinical trials, Crofelemer was the first drug approved by the FDA for the symptomatic relief of non-infectious diarrhoea in patients with HIV/AIDS on antiretroviral therapy [57c].
CLINICAL ASPECTS
Regarding its antidiarrheal effect, in 2013 a pilot study on the use of the pharmaceutical product approved by WHO "Crofelemer" (made from red latex of Croton lechleri tree) in the treatment of not infectious diarrhea was held in HIV-infected patients.
This study found that Sangre de Drago has a unique mechanism that leads to the inhibition of chloride ion secretion by blocking the chloride channels in the gastrointestinal lumen.
This reduces the flow of sodium and water, which in turn reduces the frequency and consistency of diarrhea. This drug based on Sangre de Drago is well tolerated due to minimal systemic absorption and has a good safety profile.
Therefore, it is considered important in the symptomatic relief of non-infectious diarrhea caused by antiretroviral therapy in HIV-infected people, improving their quality of life and contributing to adherence to antiretroviral therapy [58a].
Vaisberg et al. demonstrated that the topical application for a period of 17 months of either CL sap or taspine to the skin of rats is not tumorogenic [53a].
Using a mice model of wound healing, Vaisberg et al. found that taspine has a dose-response cicatrizant effect [53a]. The activity of taspine was greater than the sap and its mechanism of action might be the enhancement of fibroblasts migration [53a,b].
On the other hand, in an in vitro model Pieters et al. found that 3´,4- O-dimethylcedrusin stimulates the proliferation of endothelial cells. In this model, taspine was rather cytotoxic [41,54a].
In an in vivo model, the sap (rich in proanthocyanidins) stimulates wound contraction, formation of the crust and synthesis of collagen. The compound 3',4-O-dimethylcedrusin also improved wound healing, but was less active than the sap.
According to Pieters et al., taspine has no influence in the cicatrization even at high concentrations [54b].
In a rat model of gastric ulcers the oral treatment with CL reduced ulcer size, myeloperoxidase activity and bacterial content of the ulcer.
The expression of proinflammatory genes (TNF-, iNOS, IL1β, IL-6 y COX-2) was also reduced during SG treatment [55].
In vitro and in vivo studies demonstrate that SP-303, a large proanthocyanidin oligomer isolated from the sap of C. lechleri, has good activity against respiratory syncytial, influenza A and parainfluenza viruses.
These activities are comparable to ribavirin. SP-303 is also active against herpes virus type 1 and 2, as well against hepatitis virus A and [39b].
SP-303 in vivo decreases intestinal secretion caused by cholera toxin and in vitro reduces cAMP-mediated Cl- secretion [56a,b].
A double blind, placebo-controlled clinical trial in 169 patients with travelers´ diarrhoea showed that, compared to placebo, SP-303 was able to shorten the duration of the diarrhea by 21% [57a].
SP-303 was eventually named crofelemer and patented by Napo Pharmaceuticals. Crofelemer displays its intestinal antisecretory activity by inhibiting two secretory channels: the cystic fibrosis conductance regulator and calcium-activated chloride cannel [57b].
Following phase III clinical trials, Crofelemer was the first drug approved by the FDA for the symptomatic relief of non-infectious diarrhoea in patients with HIV/AIDS on antiretroviral therapy [57c].
CLINICAL ASPECTS
Regarding its antidiarrheal effect, in 2013 a pilot study on the use of the pharmaceutical product approved by WHO "Crofelemer" (made from red latex of Croton lechleri tree) in the treatment of not infectious diarrhea was held in HIV-infected patients.
This study found that Sangre de Drago has a unique mechanism that leads to the inhibition of chloride ion secretion by blocking the chloride channels in the gastrointestinal lumen.
This reduces the flow of sodium and water, which in turn reduces the frequency and consistency of diarrhea. This drug based on Sangre de Drago is well tolerated due to minimal systemic absorption and has a good safety profile.
Therefore, it is considered important in the symptomatic relief of non-infectious diarrhea caused by antiretroviral therapy in HIV-infected people, improving their quality of life and contributing to adherence to antiretroviral therapy [58a].
Natural Product Communications Vol. 11 (3) 2016 Lock et al. Furthermore, in 1999 a multicenter, phase II, double-blind, randomized, placebo-controlled trial evaluating the safety and efficacy of SP-303 (made from Sangre de Drago) was performed for the symptomatic treatment of diarrhea in HIV patients. HIV positive subjects were admitted to an inpatient unit of study, patients for the study discontinued all antidiarrheal agents 24 h before enrollment. Subjects in the experimental group (26 patients) received 500 mg orally every 6 hours for 96 hours (4 days), the same pattern was for the placebo group (25 patients). During the study the frequency and stool weight was evaluated. Moreover subjects were monitored for symptoms and side effects. The treatment group SP-303 showed an average reduction in stool weight baseline of 451 g/24 h versus 150 g/24 h with placebo on day 4 of treatment (p = 0.14) and a mean reduction in the frequency of abnormal stools three stools in 24 h against two stools per 24 h in the placebo group (p = 0.30). Finally, it was concluded that SP-303 is safe and well tolerated. Furthermore, these results suggest that SP-303 can be effective in reducing stool weight and frequency in patients with AIDS and diarrhea [58b]. In 1997, a multicenter double-blind, placebo-controlled, phase II study was conducted to evaluate the safety and efficacy against lesions of recurrent genital herpes in patients with AIDS. The primary endpoints of this study were the complete healing of injuries and healing time. Eligible patients had a history of genital herpes or recurrent anogenital with at least one injury. Treatment in the experimental group (24 patients) consisted of implementing the Virend® ointment three times a day for 21 days in the placebo group the same pattern (21 patients) was used. Excluding two patients in the group receiving Virend® with initial treatment, but was lost to follow up, 9 of 22 (41%) of patients treated with Virend® experienced complete healing of lesions compared with three (14%) patients in the placebo group (P = 0.053). Viral culture revealed that 50% of patients treated with Virend® and 19% of placebo treated patients became negative cultures during treatment (P = 0.06) [58c].
ECONOMIC IMPORTANCE
The initial interest of a US company for dragon’s blood croton tree sap for treating diarrhea through ethnobotanical field research got a good result because the FDA approved the First-Ever Oral Botanical Drug Amazon tree-derived medicine cleared for usage in HIV patients with diarrhea on January 2013 [59].
An extract of Croton lechleri introduced to the pharmaceutical market for use in treatment of chronic diarrhea in people living with HIV/AIDS, following the adoption of this statement represents a great market opportunity as announced by ITC [60].
However, dragon’s blood latex has been available in various products in the United States since the passage of the Dietary Supplements Health and Education Act (DSHEA) in 1994, and it is listed on the old dietary ingredients list of plants submitted by the Utah Natural Products Alliance to the US Food and Drug Administration as part of the Administration’s premarket notification program for New Dietary Ingredients [61].
In 2000, Shaman, a company that was working in reforestation of 2000 trees, licensed their dragon’s blood product to the General Nutrition Corporation (GNC), a member of the Numico family of companies, which then enabled the new product to be featured in 4,200 GNC health food stores as well as over 500 Rite AID pharmacies [62].
On the other hand, after years of research, the cosmetic application companies also include dragon's blood sap in skin elixirs, creams, and other special preparations. According to one producer, this is considered a multi-functional ingredient, being the source of many other beauty products.
ECONOMIC IMPORTANCE
The initial interest of a US company for dragon’s blood croton tree sap for treating diarrhea through ethnobotanical field research got a good result because the FDA approved the First-Ever Oral Botanical Drug Amazon tree-derived medicine cleared for usage in HIV patients with diarrhea on January 2013 [59].
An extract of Croton lechleri introduced to the pharmaceutical market for use in treatment of chronic diarrhea in people living with HIV/AIDS, following the adoption of this statement represents a great market opportunity as announced by ITC [60].
However, dragon’s blood latex has been available in various products in the United States since the passage of the Dietary Supplements Health and Education Act (DSHEA) in 1994, and it is listed on the old dietary ingredients list of plants submitted by the Utah Natural Products Alliance to the US Food and Drug Administration as part of the Administration’s premarket notification program for New Dietary Ingredients [61].
In 2000, Shaman, a company that was working in reforestation of 2000 trees, licensed their dragon’s blood product to the General Nutrition Corporation (GNC), a member of the Numico family of companies, which then enabled the new product to be featured in 4,200 GNC health food stores as well as over 500 Rite AID pharmacies [62].
On the other hand, after years of research, the cosmetic application companies also include dragon's blood sap in skin elixirs, creams, and other special preparations. According to one producer, this is considered a multi-functional ingredient, being the source of many other beauty products.
crofelmer
Crofelemer previously known as SP-303 is a large proanthocyanidin oligomer isolated from the bark latex of the plant Croton lechleri Müll. Arg.
Initial studies have demonstrated the immense antiviral activity of crofelemer against a gamma of DNA and RNA viruses such as respiratory syncytial virus, influenza A virus, parainfluenza virus, herpesvirus types 1 and 2, and hepatitis A and B viruses. The antiviral mechanism implies the direct interaction of crofelemer to components of the viral envelope, blocking both the viral attachment and the cell invasion.
More recently, crofelemer is used as a first-in-class antidiarrheal medication, and its efficacy has been investigated in vivo assays [42] and in patients with HIV-associated diarrhea, diarrhea of various infectious etiologies, as well as diarrhea-predominant irritable bowel syndrome.
Crofelemer was recently approved by the FDA to treat diarrhea in HIV/AIDS patients on antiretroviral therapy.
The mechanism of action as antidiarrheal of this proanthocyanidin oligomer consists in the dual inhibitory action on two structurally unrelated prosecretory intestinal Cl− channels, which are responsible for chloride secretion and subsequent luminal hydration. The first target is an extracellular site of the cystic fibrosis transmembrane regulator (CFTR) Cl− channel (∼60%, IC50 ∼ 7 μM), which produces a voltage-independent block with stabilization of the channel closed state. The second target is the intestinal calcium-activated Cl− channel (CaCC) by a voltage-independent inhibition mechanism (>90%, IC50 ∼ 6.5 μM).
Initial studies have demonstrated the immense antiviral activity of crofelemer against a gamma of DNA and RNA viruses such as respiratory syncytial virus, influenza A virus, parainfluenza virus, herpesvirus types 1 and 2, and hepatitis A and B viruses. The antiviral mechanism implies the direct interaction of crofelemer to components of the viral envelope, blocking both the viral attachment and the cell invasion.
More recently, crofelemer is used as a first-in-class antidiarrheal medication, and its efficacy has been investigated in vivo assays [42] and in patients with HIV-associated diarrhea, diarrhea of various infectious etiologies, as well as diarrhea-predominant irritable bowel syndrome.
Crofelemer was recently approved by the FDA to treat diarrhea in HIV/AIDS patients on antiretroviral therapy.
The mechanism of action as antidiarrheal of this proanthocyanidin oligomer consists in the dual inhibitory action on two structurally unrelated prosecretory intestinal Cl− channels, which are responsible for chloride secretion and subsequent luminal hydration. The first target is an extracellular site of the cystic fibrosis transmembrane regulator (CFTR) Cl− channel (∼60%, IC50 ∼ 7 μM), which produces a voltage-independent block with stabilization of the channel closed state. The second target is the intestinal calcium-activated Cl− channel (CaCC) by a voltage-independent inhibition mechanism (>90%, IC50 ∼ 6.5 μM).