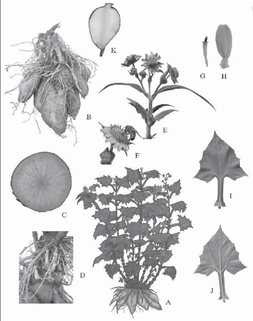
Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson, Class Dicot, Order Asterales Family Asteraceae.
The cultivation of Yacon dates from Pre-Inca age (Nazca, Paracas and Mochica cultures) so the historical use of this species is directly related to "traditional knowledge" – that was possessed by native Indian people, AfroAmerican and local communities, and transmitted from one generation to the other, usually orally and outside the formal education system.
The cultivation of Yacon dates from Pre-Inca age (Nazca, Paracas and Mochica cultures) so the historical use of this species is directly related to "traditional knowledge" – that was possessed by native Indian people, AfroAmerican and local communities, and transmitted from one generation to the other, usually orally and outside the formal education system.
|
SCIENTIFIC NAME
Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson SYNONYM Polymnia sonchifolia Poepp. & Endl. 1845 - Polymnia edulis Wedd. 1857 COMMON NAMES Llacon: North of Peru - Llakwash: Ferreñafe, Lambayeque in Peru - Aricoma: Aymara - Aricuma: Quechua - Jicama: Ecuador, Venezuela, Colombia - Xicama: Mexico, Peru |
PLANT MORPHOLOGY
Habit: herbaceous, perennial, erect, 0.8 to 2 m long, with few to many branches. Roots: storage roots characterized by accumulation of fructose polymer in the parenchymatous tissue. At the top, crown buds used in plant propagation. Stems: cylindrical, pubescent, varying from green to purple, hollow at maturity branched or not, depending on whether it was reproduced vegetatively or by seed. Leaves: petiolate, opposite decussate, triangular blade, edge irregularly dentate, acute apex, base hastate, with three prominent nervous, slightly pubescent on the adaxial face different from abaxial face which have a great pubescence. Difference is obvious leaf size before and after flowering, being smaller thereafter. Inflorescence and flowers: capitulum arranged in dichasia. Unisexual flowers, being female ligulate and male flowers tubular, both corollas are gamopetalous with 5 petals fused; female flowers have inferior ovary, fusiform and purple; male stamens with fused anthers, which are black. Fruit: typical in family Asteraceae, that is a Cypsela. Habitat: it can be found both cultivated and in wild forms, in earthy sandy clay soil and an elevation between 50-3500 m altitude , from Venezuela and Colombia to northern Argentina. In recent years, its cultivation has acquired great importance because of its medicinal properties, so this activity is well widespread not only in Latin America but also in Europe and Asia.
ETHNOMEDICINE
Through the years, Yacon has been used as an excellent product to satisfy hunger and thirst, as well as for its various therapeutic effects that have been passed down for generations. Both the leaves and fruits are used. The latter is traditionally used as fresh or dried fruit at different degrees, and occasionally (for ceremonies or parties) as chicha or jam. The fruit is used for rehydratation due to its high water content, it prevents fatigue and cramps. In addition, it is also used to prevent rickets and for kidney and liver conditions. In Bolivia, the use of the leaves by diabetics and for digestion conditions has been reported; in the north of Peru, it is traditionally eaten before going to bed to delay aging. Furthermore, it is indicated to relieve constipation, lower high-blood pressure, prevent colon cancer and as antimicrobial and antiparasitic.
CHEMICAL CONSTITUENTS
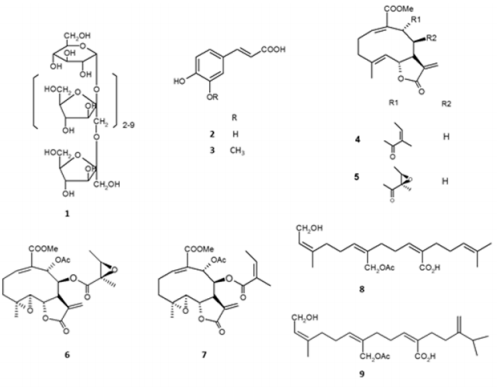
The key component of yacon roots is composed of oligosaccharides, specifically the fructo-oligosaccharides, FOS, also known as oligofructans or oligofructoses, belonging to the fructans. FOS are made up of fructose units connected by β (2 → 1) and/or β (6 → 1) links [13a]. FOS (1), consist of 3 to 10 fructose units and they always contain a glucose unit at the start of each fructan chain with a α (1→2) link. It is estimated that 50-70% of the dried root is composed by FOS, as opposed to other roots whose main component is starch [13b]. Also present in yacon roots are phenolic compounds. In addition to known acids like caffeic (2), chlorogenic, and ferulic (3) acids, three new caffeoyl esters of atraric acid, as well as two caffeoyl esters (mono and dicaffeoyl esters of octulosonic acid) have been reported. The latter is classified as a keto-aldonic acid, with an skeleton 6, 8-[3,2,1] octane, a structure that is rarely found in natural products [14 a-d]. Other chemical constituents present in small quantities in the roots of yacon are proteins, fats, fiber, glucose, fructose, saccharose; and other nutrients like calcium, phosphorous, iron, niacin, riboflavin, ascorbic acid and the Ltryptophan amino acid [15]. From the leaves of yacon, a new melampolide- type sesquiterpene lactone, called sonchifolin, was isolated (4), as well as the wellknown polymatin B, uvedalin (5), enhydrin (6), and fluctuanin (7), in addition to two other structures recently reported: the methyl esters of the acids 8β- tigloyloxymelampolid-14-oic and 8βmetacryloyloxymelampolid-14-oic [16a,b]. Diterpenoid compounds present are the ent-kaurenoic acid and its angeloyloxide derivatives [16c] two new diterpenes tetrahidroxy ent-kauranes named entkaurane-3β,16β,17,19-tetrol and ent-kaurane-16β,17,18,19-tetrol [16d] and new acyclic diterpenic acids called smaditerpenic acids A-D [16e] and E-F (8-9) [16f] (Figure 1). Amongst the phenolic compounds present in the leaves are the gallic, chlorogenic, caffeic and ferulic acids; as well as the flavonoids rutin, myricetin, kaempferol and quercetin; being gallic acid and
The key component of yacon roots is composed of oligosaccharides, specifically the fructo-oligosaccharides, FOS, also known as oligofructans or oligofructoses, belonging to the fructans. FOS are made up of fructose units connected by β (2 → 1) and/or β (6 → 1) links [13a]. FOS (1), consist of 3 to 10 fructose units and they always contain a glucose unit at the start of each fructan chain with a α (1→2) link. It is estimated that 50-70% of the dried root is composed by FOS, as opposed to other roots whose main component is starch [13b]. Also present in yacon roots are phenolic compounds. In addition to known acids like caffeic (2), chlorogenic, and ferulic (3) acids, three new caffeoyl esters of atraric acid, as well as two caffeoyl esters (mono and dicaffeoyl esters of octulosonic acid) have been reported. The latter is classified as a keto-aldonic acid, with an skeleton 6, 8-[3,2,1] octane, a structure that is rarely found in natural products [14 a-d]. Other chemical constituents present in small quantities in the roots of yacon are proteins, fats, fiber, glucose, fructose, saccharose; and other nutrients like calcium, phosphorous, iron, niacin, riboflavin, ascorbic acid and the Ltryptophan amino acid [15]. From the leaves of yacon, a new melampolide- type sesquiterpene lactone, called sonchifolin, was isolated (4), as well as the wellknown polymatin B, uvedalin (5), enhydrin (6), and fluctuanin (7), in addition to two other structures recently reported: the methyl esters of the acids 8β- tigloyloxymelampolid-14-oic and 8βmetacryloyloxymelampolid-14-oic [16a,b]. Diterpenoid compounds present are the ent-kaurenoic acid and its angeloyloxide derivatives [16c] two new diterpenes tetrahidroxy ent-kauranes named entkaurane-3β,16β,17,19-tetrol and ent-kaurane-16β,17,18,19-tetrol [16d] and new acyclic diterpenic acids called smaditerpenic acids A-D [16e] and E-F (8-9) [16f] (Figure 1). Amongst the phenolic compounds present in the leaves are the gallic, chlorogenic, caffeic and ferulic acids; as well as the flavonoids rutin, myricetin, kaempferol and quercetin; being gallic acid and
Figure 1: Some chemical compounds isolated from Smallanthus sonchifolius.
PHARMACOLOGY
The methanol extract of yacon roots showed good antioxidant activity in the DPPH test. The phenolic compounds caffeic and chlorogenic acids would be responsible for this activity.
Sonchifolin, a melampolide isolated from the methanolic extract of the leaves of yacon possess good activity against the fungus Pyricularia oryzae; while the melampolides fluctuanin, uvedalin and enhydrin were active against the bacterium Bacillus subtilis [16a,b]. Ent-kaurenoic acid from the dichloromethane extract of yacon leaves is active against Staphylococcus aureus and S. epidermidis [19a]. Enhydrin was even active against methicillinresistant S. aureus [19b]. Enhydrin and uvedalin might have potential as agents against Chagas disease, since they have significant trypanocidal activity against the trypomastigote forms of Trypanosoma cruzi [19c]. The topical application (0.25 mg/ear) of yacon leaves extract, rich in sesquiterpenelactones, reduced the inflammation by 44.1% compared to the control group [20]. Aybar et al. demonstrated that a decoction of yacon leaves have a hypoglycemic effect in rats with diabetes induced by streptozotocin (STZ), with a concomitant increase in circulating insulin concentration [21a]. Aqueous extract and the ethylacetate fraction decrease the glucose production in normal hepatocytes. This effect could be explained by an increase of insulin synthesis and/or the inhibition of hepatic gluconeogenesis and glycogenolysis [21b, c]. The sesquiterpene lactone enhydrin, the diterpene ent-kaurenoic acid and a butanol extract from the leaves of yacon, rich in caffeic, chlorogenic and three dicaffeoilquinic acids, showed good in vivo hypoglycemic activity [21d,e]. According to Genta et al., these compounds are responsible for the hypoglycemic activity of yacon leaves, although their mechanisms of action are still unknown [21d]. Other coumpounds that could be also responsible for the antidiabetes activity of yacon leaves are the smallanthaditerpenic acids (A – D); all of them exhibited in vitro -glucosidase inhibitory activity [21f]. The roots of yacon also exhibit antidiabetes activity in vivo. An aqueous extracts of the roots reversed dyslipidaemia and hyperglycaemia in rats with diabetes mellitus (DM) induced by STZ. It also showed a hepatoprotective effect and an improvement of symptoms commonly associated with DM type 1 (hyperphagia, polydipsia and weight loss) [22a]. Yacon root extracts and one of its constituents (chlorogenic acid) produced a significant hypoglycemic effect in STZ-induced diabetic rats. Also, they decreased total cholesterol and triglyceride concentrations [22b]. The fructooligosaccharides (FOS) present in yacon roots behave as prebiotics by promoting the growth of probiotic organisms. Pedrechi et al. demonstrated that FOS of yacon are metabolized by 3 strains of known probiotics (Lactobacillus acidophilus NRRL1910, Lactobacillus plantarum NRRL B-4496 y el Bifidobacterium bifidum ATCC 15696) [13b]. Yacon roots flour has a prebiotic effect in vivo by stimulating the growth of bifidobacteria and lactobacilli, and by increasing the concentrations of short chain fatty acids [23].
CLINICAL ASPECTS
There are no clinical studies on safety and tolerance, however, some pilot studies have been reported, the same being aimed at proving the hypoglycemic effect and prebiotic action of both the leaves and fruits of Smallanthus sonchifolius. Regarding the use of the leaves, there is a clinical trial conducted in 206 adults who were divided into two groups: one experimental group (diabetic patients on glibenclamide) which was subdivided into two subgroups, one on glibenclamide and the other on glibenclamide plus yacon. The other group was the control group made up of apparently healthy people; it was subdivided into two subgroups, one group receiving no treatment and the other only yacon leaves. The plant was prepared as an infusion, with 1g of yacon leaves in teabags, to be drunk 3 times daily. All groups were evaluated before and after treatment forBody Mass Index (BMI), fasting blood glucose, glycosylated hemoglobin (HbA1c) and fructosamine. It was observed that in the subgroups who were given yacon leaves, fasting blood glucose decreased by 42.7%, glycosylated HbA1c by 21.7%, and fructosamine by 33.78% [24a]. With respect to the use of the fresh root of Samallanthus sonchiofolius, there is an unblinded pilot clinical trial with a before and after design, involving 6 apparently healthy subjects whose BMI was 21.75. All subjects underwent biochemical tests (liver function and lipid profile, complete blood count), in addition, all received Oral Glucose Tolerance Test (OGTT). The results were within the normal range. Next, they were administered 300 g of fresh yacon root orally and received OGTT, the results showed a reduction of 79.8% (p = 0.001) in postprandial glycemic response [24b]. Furthermore, a comparative clinical trial of the effect of the leaves and fresh root of Samallanthus sonchiofolius on seric glucose and glycosilated hemoglobin was conducted on 30 patients with type II diabetes receiving pharmacological treatment and with an ad libitum diet. These patients were divided into 3 groups: the first group received 500 g/day of yacon fresh root; the second group, lyophilized yacon extract equivalent to 500 g/day of the fresh fruit; and the third group was given yacon-leaf teabags (each teabag equivalent to 1g of leaves) to drink three times per day. With respect to HbA1c, an average decrease of 1.98%, 1.84% and 1.14% was observed in each group, respectively; and seric glucose decreased considerably, in greater extent in the third group and in lesser extent in the fresh fruit group [24c]. Another important characteristic of yacon is its prebiotic effect. In order to evaluate this characteristic, a study was conducted with 16 healthy subjects (8 men and 8 women) who received 20 g of fresh yacon (equivalent to 6.4 g of FOS) daily. A two-week crossover design was used. The evaluation measured the colonic transit time using radio-opaque markers, and showed that the time reduced significantly from 59.7 +/- 4.3 to 38.4 +/- 4.2 hours with p< 0.0001. The frequency of bowel movement increased from 1.1 to 1.3. Very few adverse effects were observed, only a slight increase in meteorism. The study concluded that yacon accelerates intestinal transit significantly [25].
ECONOMIC IMPORTANCE
The first introduction of yacon in Europe was made in 1927 by Calvino, with the aim of finding an alternative fuel (alcohol) and development of forage production in Northern Italy. After adaptation research, it was recommended to use yacon as a nutrition source, as a feeding crop, and mainly, as a material for sugar industry. A couple years later, yacon was introduced in Germany in 1941, in Hamburg and Wulfsdorf. Yacon was also introduced in the Czech Republic, where it has been grown since 1994 [26]. In the 80s, it entered New Zealand as a new crop [27]. However, it has not been demanded directly as a commercial vegetable. During the last thirty years, yacon was again enhanced in a production of processed foods, extracts and syrups. In New Zealand, the commercialization of novel foods is regulated by the Standard 1.5.1 of the Australia New Zealand Food Standards Code. Yacon is valued in Japan and Korea as food and Food Ingredient and it is used in a variety of products. In addition, the U.S. Environmental Protection Agency (EPA) includes yacon in its lists of food crops for purposes of establishing pesticide residue tolerances [28]. In Canada, yacon root extract is permitted for use as a non-medicinal sweetening agent [29]. In European market, the situation of yacon changed at the beginning of 2014. A history of significant food use of yacon roots or Smallanthus sonchifolius in the EU before 1997 has been demonstrated, and thus it is no longer considered novel food [30]. In the meantime, the Peruvian Anti-Biopiracy Commission has the task of developing actions to identify, prevent and avoid acts of biopiracy with the aim of protecting the interests of the Peruvian State [31]. Until now, the Peruvian National Commission against Biopiracy found 2800 patents applications. Japan is the country that has researched yacon for more than 10 years. The Peruvian Commission against Bio-piracy mentions 50 Japanese patents involving yacon, in some of which it constitutes the primary component of the invention [32]. Mainly, yacon patents refer to preparation as food, pharmaceuticals and cosmetics ingredients uses.
The methanol extract of yacon roots showed good antioxidant activity in the DPPH test. The phenolic compounds caffeic and chlorogenic acids would be responsible for this activity.
Sonchifolin, a melampolide isolated from the methanolic extract of the leaves of yacon possess good activity against the fungus Pyricularia oryzae; while the melampolides fluctuanin, uvedalin and enhydrin were active against the bacterium Bacillus subtilis [16a,b]. Ent-kaurenoic acid from the dichloromethane extract of yacon leaves is active against Staphylococcus aureus and S. epidermidis [19a]. Enhydrin was even active against methicillinresistant S. aureus [19b]. Enhydrin and uvedalin might have potential as agents against Chagas disease, since they have significant trypanocidal activity against the trypomastigote forms of Trypanosoma cruzi [19c]. The topical application (0.25 mg/ear) of yacon leaves extract, rich in sesquiterpenelactones, reduced the inflammation by 44.1% compared to the control group [20]. Aybar et al. demonstrated that a decoction of yacon leaves have a hypoglycemic effect in rats with diabetes induced by streptozotocin (STZ), with a concomitant increase in circulating insulin concentration [21a]. Aqueous extract and the ethylacetate fraction decrease the glucose production in normal hepatocytes. This effect could be explained by an increase of insulin synthesis and/or the inhibition of hepatic gluconeogenesis and glycogenolysis [21b, c]. The sesquiterpene lactone enhydrin, the diterpene ent-kaurenoic acid and a butanol extract from the leaves of yacon, rich in caffeic, chlorogenic and three dicaffeoilquinic acids, showed good in vivo hypoglycemic activity [21d,e]. According to Genta et al., these compounds are responsible for the hypoglycemic activity of yacon leaves, although their mechanisms of action are still unknown [21d]. Other coumpounds that could be also responsible for the antidiabetes activity of yacon leaves are the smallanthaditerpenic acids (A – D); all of them exhibited in vitro -glucosidase inhibitory activity [21f]. The roots of yacon also exhibit antidiabetes activity in vivo. An aqueous extracts of the roots reversed dyslipidaemia and hyperglycaemia in rats with diabetes mellitus (DM) induced by STZ. It also showed a hepatoprotective effect and an improvement of symptoms commonly associated with DM type 1 (hyperphagia, polydipsia and weight loss) [22a]. Yacon root extracts and one of its constituents (chlorogenic acid) produced a significant hypoglycemic effect in STZ-induced diabetic rats. Also, they decreased total cholesterol and triglyceride concentrations [22b]. The fructooligosaccharides (FOS) present in yacon roots behave as prebiotics by promoting the growth of probiotic organisms. Pedrechi et al. demonstrated that FOS of yacon are metabolized by 3 strains of known probiotics (Lactobacillus acidophilus NRRL1910, Lactobacillus plantarum NRRL B-4496 y el Bifidobacterium bifidum ATCC 15696) [13b]. Yacon roots flour has a prebiotic effect in vivo by stimulating the growth of bifidobacteria and lactobacilli, and by increasing the concentrations of short chain fatty acids [23].
CLINICAL ASPECTS
There are no clinical studies on safety and tolerance, however, some pilot studies have been reported, the same being aimed at proving the hypoglycemic effect and prebiotic action of both the leaves and fruits of Smallanthus sonchifolius. Regarding the use of the leaves, there is a clinical trial conducted in 206 adults who were divided into two groups: one experimental group (diabetic patients on glibenclamide) which was subdivided into two subgroups, one on glibenclamide and the other on glibenclamide plus yacon. The other group was the control group made up of apparently healthy people; it was subdivided into two subgroups, one group receiving no treatment and the other only yacon leaves. The plant was prepared as an infusion, with 1g of yacon leaves in teabags, to be drunk 3 times daily. All groups were evaluated before and after treatment forBody Mass Index (BMI), fasting blood glucose, glycosylated hemoglobin (HbA1c) and fructosamine. It was observed that in the subgroups who were given yacon leaves, fasting blood glucose decreased by 42.7%, glycosylated HbA1c by 21.7%, and fructosamine by 33.78% [24a]. With respect to the use of the fresh root of Samallanthus sonchiofolius, there is an unblinded pilot clinical trial with a before and after design, involving 6 apparently healthy subjects whose BMI was 21.75. All subjects underwent biochemical tests (liver function and lipid profile, complete blood count), in addition, all received Oral Glucose Tolerance Test (OGTT). The results were within the normal range. Next, they were administered 300 g of fresh yacon root orally and received OGTT, the results showed a reduction of 79.8% (p = 0.001) in postprandial glycemic response [24b]. Furthermore, a comparative clinical trial of the effect of the leaves and fresh root of Samallanthus sonchiofolius on seric glucose and glycosilated hemoglobin was conducted on 30 patients with type II diabetes receiving pharmacological treatment and with an ad libitum diet. These patients were divided into 3 groups: the first group received 500 g/day of yacon fresh root; the second group, lyophilized yacon extract equivalent to 500 g/day of the fresh fruit; and the third group was given yacon-leaf teabags (each teabag equivalent to 1g of leaves) to drink three times per day. With respect to HbA1c, an average decrease of 1.98%, 1.84% and 1.14% was observed in each group, respectively; and seric glucose decreased considerably, in greater extent in the third group and in lesser extent in the fresh fruit group [24c]. Another important characteristic of yacon is its prebiotic effect. In order to evaluate this characteristic, a study was conducted with 16 healthy subjects (8 men and 8 women) who received 20 g of fresh yacon (equivalent to 6.4 g of FOS) daily. A two-week crossover design was used. The evaluation measured the colonic transit time using radio-opaque markers, and showed that the time reduced significantly from 59.7 +/- 4.3 to 38.4 +/- 4.2 hours with p< 0.0001. The frequency of bowel movement increased from 1.1 to 1.3. Very few adverse effects were observed, only a slight increase in meteorism. The study concluded that yacon accelerates intestinal transit significantly [25].
ECONOMIC IMPORTANCE
The first introduction of yacon in Europe was made in 1927 by Calvino, with the aim of finding an alternative fuel (alcohol) and development of forage production in Northern Italy. After adaptation research, it was recommended to use yacon as a nutrition source, as a feeding crop, and mainly, as a material for sugar industry. A couple years later, yacon was introduced in Germany in 1941, in Hamburg and Wulfsdorf. Yacon was also introduced in the Czech Republic, where it has been grown since 1994 [26]. In the 80s, it entered New Zealand as a new crop [27]. However, it has not been demanded directly as a commercial vegetable. During the last thirty years, yacon was again enhanced in a production of processed foods, extracts and syrups. In New Zealand, the commercialization of novel foods is regulated by the Standard 1.5.1 of the Australia New Zealand Food Standards Code. Yacon is valued in Japan and Korea as food and Food Ingredient and it is used in a variety of products. In addition, the U.S. Environmental Protection Agency (EPA) includes yacon in its lists of food crops for purposes of establishing pesticide residue tolerances [28]. In Canada, yacon root extract is permitted for use as a non-medicinal sweetening agent [29]. In European market, the situation of yacon changed at the beginning of 2014. A history of significant food use of yacon roots or Smallanthus sonchifolius in the EU before 1997 has been demonstrated, and thus it is no longer considered novel food [30]. In the meantime, the Peruvian Anti-Biopiracy Commission has the task of developing actions to identify, prevent and avoid acts of biopiracy with the aim of protecting the interests of the Peruvian State [31]. Until now, the Peruvian National Commission against Biopiracy found 2800 patents applications. Japan is the country that has researched yacon for more than 10 years. The Peruvian Commission against Bio-piracy mentions 50 Japanese patents involving yacon, in some of which it constitutes the primary component of the invention [32]. Mainly, yacon patents refer to preparation as food, pharmaceuticals and cosmetics ingredients uses.